Abstract
Hydrogen sulfide (H2S) serves as a gasotransmitter in the regulation of organ development and maintenance of homeostasis in tissues. Its abnormal levels are associated with multiple human diseases, such as neurodegenerative disease, myocardial injury, and ophthalmic diseases. Excessive exposure to H2S could lead to cellular toxicity, orchestrate pathological process, and increase the risk of various diseases. Interestingly, under physiological status, H2S plays a critical role in maintaining cellular physiology and limiting damages to tissues. In mammalian species, the generation of H2S is catalyzed by cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), 3-mercapto-methylthio pyruvate aminotransferase (3MST) and cysteine aminotransferase (CAT). These enzymes are found inside the mammalian eyeballs at different locations. Their aberrant expression and the accumulation of substrates and intermediates can change the level of H2S by orders of magnitude, causing abnormal structures or functions in the eyes. Detailed investigations have demonstrated that H2S donors’ administration could regulate intraocular pressure, protect retinal cells, inhibit oxidative stress and alleviate inflammation by modulating the function of intra or extracellular proteins in ocular tissues. Thus, several slow-releasing H2S donors have been shown to be promising drugs for treating multiple diseases. In this review, we discuss the biological function of H2S metabolism and its application in ophthalmic diseases.
Similar content being viewed by others
Facts as indicated in the Instructions
-
H2S is not only a poisonous gas, but also has critical role in maintaining homeostasis and functions of eye.
-
H2S is endogenously generated and serves as a gaseous modulator in eye.
-
H2S shows diverse effects on ocular tissues in both physiological or pathological situations, which are mostly influenced by its concentration.
Open Questions
-
The beneficial/toxic concentrations of H2S have not been established in different tissues.
-
The most effective administrative route of H2S for different eye diseases needs to be determined.
-
Drugs that can be co-administered with H2S for congenital ophthalmic diseases have not been determined.
Introduction
Hydrogen sulfide (H2S) was identified by Carl Wilhelm Scheele through chemical analysis in the 17th century. However, it has long been believed that this gas emanated from the sewer system is related to a series of a special type of eye diseases occurred in sewer workers. This disease is associated with painful inflammation, secondary bacterial invasion and even blindness. Like nitric oxide (NO) and carbon monoxide, endogenously produced H2S is now known as another gaseous signaling molecule that affects the structure and function of proteins by participating in their short-lived covalent reactions1. This gasotransmitter can easily diffuse across cell membranes and does not need a specific mechanism for their degradation and reuptake. In human, the concentration of H2S in tissues can be at μM ranges for maintaining the physiological cellular functions. Its levels can differ according to age, tissues and measuring methods2,3. For example, the H2S concentration in the peripheral blood is generally 30–300 μM4, while the physiological concentration of H2S in the brain is up to three times of that in serum5,6. The H2S gas/water coefficient of distribution is 0.39, which can be affected by pH2,7. In comparison to healthy individuals, the H2S concentration in the serum of asthmatic patients can reach to 600 μM8.
The oxidation products of H2S include persulfide, sulfite, thiosulfate and sulfate9. When the concentrations of H2S in tissues or cells are high, H2S is considered as a toxic substance and its oxidation products may cause cytotoxic effects through inhibiting mitochondrial cytochrome C oxidase and disrupting cell energy production, leading to tissue inflammation or DNA damage10. However, when it is generated at physiological rates or at low concentrations, it has entirely different effects on biological processes such as cellular division, DNA repair and metabolism, modulation of protein kinase, regulation of cell cycle and organization of cytoskeletal framework11. Recent investigations have found that the potential regulatory role of H2S is to add cysteine, a thiol group in proteins (aka S-sulfhydration, or persulfide formation)12. This modification critically changes the physiological actions and pathological status of proteins in response to inflammation or oxidation by generating a –SSH group. The persulfides have better reactivity than corresponding thiols and can readily react with electrophiles. Persulfidation of proteins such as KATP contributes to various H2S-induced biochemical reactions13. When H2S is produced at low levels through enzymatical degradation of cysor homocysteine, it is critical in maintaining the functions of nervous system and vascular system14,15. Exogenously administrated H2S has been found to extend the lifespan of worms, relieve inflammation and promote reparation of injured tissues16. In views of the potential value of H2S in body systems and its presence in mammalian eyes17, this review focuses on the role of H2S in the common ophthalmic diseases and the underlying mechanisms, hoping to provide therapeutic strategy for ophthalmic diseases. Detailed analysis on the crosstalk between ocular tissues and H2S generation pathway will pave the road for understanding the pathogenesis of multiple ophthalmic diseases and optimize the application of H2S donor for treatment.
Generation of H2S in ocular tissues
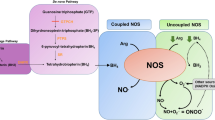
In mammalian cells, the generation of H2S is dependent on four major enzymes: cystathionine-γ-synthase (CSE), cystathionine-β-lyase (CBS), 3-mercapto-methylthio pyruvate aminotransferase (3MST) and cysteine aminotransferase (CAT)18,19. Generally, the generation of H2S relies on the desulfurization of cysteine or homocysteine by the two pyridoxial 5-phosphate (PLP)-dependent enzymes, CSE and CBS7. Of note, the distribution of these enzymes for H2S production shows tissue specificity. For example, CBS is the main enzyme for H2S generation in the central nervous system20. CSE is the major enzyme for H2S production in the vasculature system, liver, and kidney21,22,23. The presence of these H2S-productive enzymes are proved in ocular tissues, especially in the retina24,25,26. According to recent studies, H2S can also be produced from D-cysteine, catalyzed by D-Amino acid oxidase (DAO) and 3MST27.
Endogenously production of H2S is discovered in various tissues of bovine eye, including cornea, aqueous humor, iris, ciliary muscle, lens, choroid and retina, except vitreous humor. The highest production of endogenous H2S was detected in cornea and retina17. CBS is most highly expressed in the cornea, conjunctiva, and iris, while much lower amount be found in retina and optic nerve, relatively lower amount in lens, but absent in the vitreous humor. CBS expression remains high in anterior segments throughout the lifespan, and it has a trend of age-dependent increase in retina24. CSE is characterized in retina of amphibians and mammals, where its activity can be traced25. 3MST/CAT pathway is the dominating way to produce H2S in mammalian retina as both 3MST and CAT are located in the retinal neurons, which is increased at low concentrations of Ca2+ that achieved in brightness26. Deficiency of H2S or its substrates are found to be related to ectopialentis, myopic, cataract28, optic atrophy, and retinal detachment29,30,31 (Fig. 1).
H2S generation is mainly controlled by four enzymes, CBS (major source of H2S production in central nervous system), CSE (major source of H2S production in vasculature system, liver and kidney), 3MST and CAT (major source of H2S production in retina). CSE and CAT are regulated by Ca2+. H2S is produced by these enzymes at steady-state low intracellular concentrations of Ca2+. Multiple ocular tissues showed the presence of endogenous H2S, including lens, iris, choroid, ciliary muscle, aqueous humor, cornea, and retina, except vitreous humor. The highest concentrations of endogenous H2S are detected in cornea and retina, of which the production differs in their major enzymes
H2S and glaucoma
Reduction of intraocular pressure (IOP)
High IOP is the major cause for optic neuropathy in patients of glaucoma, which damages the retinal neurons and optic nerve heads32. Stable IOP depends on the balance of aqueous humor (AH) generation in the ciliary body and AH outflow in the chamber angle, especially in trabecular meshwork33. The outflow facility could be increased by cyclic adenosine monophosphate (cAMP) administration in anterior chamber for the maintenance of IOP34. H2S-releasing compounds could act on adenylyl cyclase and ATP-sensitive potassium channels (KATP) channels in eyes, thus increase cAMP concentrations in porcine ocular anterior segments and help mediate the outflow of AH35. Ex vivo study has indicated that H2S participates in the phosphodiesterase (PDE) inhibition and enhancement of intramitochondrial cAMP levels, which stimulates protein kinase A (PKA) to instruct bioenergetic effects36. The inhibition of PDE activity by H2S is a relevant factor to cumulative cAMP and cyclic guanosine monophosphate (cGMP). Meanwhile, elevated intraocular cGMP level is related to reducing the trabecular meshwork cell volume and promoting outflow of AH37. H2S-producing donors such as GYY4137, are well-investigated for stabilizing IOP, as their administration upregulate the intraocular glutathione (GSH) expression with increased cGMP levels38,39.
H2S donors also work on anterior uvea to relax iris smooth muscles40 and thus lower IOP. On the other hand, norepinephrine released by intraocular degenerating sympathetic nerve terminals can cause a decrease in outflow facility with a subsequent elevated IOP in the long term, even though it may lead to an acute increase in outflow facility41. Increased levels of norepinephrine in AH during night is related to an increase rather than a decrease in IOP in rabbits42. H2S can reduce the release of norepinephrine from sympathetic nerves43, which contributes to stabilizing IOP.
Effect on ocular blood supply
Ischemia can cause glaucomatous damage accompany with or without an abnormal IOP. In vivo studies have revealed that inadequate blood supply can lead to optic nerve head atrophy and cell death in ganglions, which implies that abnormal ocular blood flow (OBF) necessarily affects metabolic processes to adapt to visual function needs44.
Several conflicting reports are published about the pharmacological reactions of H2S in vasculature of diverse organs in different species. It is reported that high concentrations of GYY4137 (1 mM) can significantly raise phenylephrine-induced tone in the ophthalmic arteries of rabbits45, but more evidences have proven that newly derived H2S donors exert vasodilator effects on pre-contracted posterior ciliary arteries (PCAs)46,47, which are crucial to OBF. Low concentrations of GYY4137 (100 nM–100 μM) may elicit relaxations in PCAs in the presence of phenylephrine induced tone via endogenous production of both prostanoids and H2S47. AP72 and AP67 show vasodilation effect on phenylephrine-induced PCAs in a concentration-dependent manner46. These effects are mainly dependent upon the action on KATP channels by H2S. Taken together, these studies have established the role of H2S in modulating the OBF of glaucoma.
Protection on neurons
The major features of glaucoma include progressive cell death of retinal ganglions and optic nerve damage48,which are usually induced by loss of neurotrophic factors, intracellular and extracellular toxicity of glutamate, and neuro-inflammation48,49,50,51,52. In the nervous system, H2S functions as neurotransmitters53 and possesses the ability to inhibit apoptosis and degradation of neurons54. H2S produced by astrocytes acts as a synaptic modulator and causes excitation to nearby neurons by controlling calcium ion influx of astrocytes55. For eyes, in vitro experiments have demonstrated that addition of H2S donors to the culture system effectively inhibits the release of sympathetic neurotransmission from isolated bovine iris-ciliary bodies56, and inhibits amino acid neurotransmission in isolated bovine retina57, which is mediated by its action on the KATP channels or NO synthase.
H2S can not only enhances the N-methyl-D-aspartate (NMDA) receptor-mediated responses in physiological concentrations20, but also modulates the over-activated NMDA receptors via the cAMP axis58,59,60. Aberrant metabolism or signaling pathways of H2S are found in various neurodegenerative diseases, such as declined levels of H2S in Alzheimer’s patients61, impaired CSE transcription in Huntington’s disease62, depleted sulfhydration in Parkinson’s disease63, and increased H2S levels found in amyotrophic lateral sclerosis64. The fact that H2S modulates cell functions, protects neurons from apoptosis or oxidative stress are widely confirmed65,66,67. H2S is able to neutralize excess peroxynitrite (ONOO−) or other free radicals, to antagonize lipid peroxidation and oxidation of thiols, and to reverse mitochondrial dysfunction7. It works as an anti-oxidant for eliminating the excessive glutamate together with glutathione68, as well as activating KATP channels to combat oxidative glutamate toxicity69. H2S could inhibit the generation of reactive oxygen species (ROS)70 and ameliorate the toxic effect of hypochlorous acid (HOCl) generated from myeloperioxidase (MPO) catalysis, thereby exerting anti-oxidant effects and protecting neuronal cells from cellular chlorinative damage71. H2S presents anti-apoptotic effect on the SH-SY5Y cell line in low concentrations by preserving mitochondrial functions, which is referred to suppressing cytochrome oxidase C and opening the mitochondrial KATP channels72.
Referring to the anti-oxidant activity by H2S donors exerted on neurons, studies have found that H2S could increase the GSH concentration in neurons by enhancing the transporter of cysteine, cysteine/glutamate antiporter and γ-glutamyl cysteine synthetase (γ-GCS)73,74. γ-GCS and GSH synthetase act concertedly during the synthesis of GSH. Both enzymes can be regulated by Nrf2, which is also one potential targets of H2S75. The consequence of H2S-regulated Nrf2 pathway in neurons is to enhance the expression of glutathione-S-transferase (GST) and heme oxygenase (HO-1), the oxidative stress-related antioxidant enzymes76. ACS14 and ACS1, two donors of H2S, are confirmed to improve the intracellular GSH level and promote neuroprotective effects via opening KATP channels77.
H2S could promote cell survival through effectively activating protein kinase C-α (PKC-α), inhibiting NF-κΒ signaling pathway, as well as upregulating Bcl-2 and X chromosome-linked inhibitor of apoptosis (XIAP) levels in RGC cells that pre-treated with glutamate (Glu) and buthionine sulfoximine (BSO)76. In comparison with glutamate treated RGC cells, addition of H2S enhances Akt phosphorylation and promotes cell viability in response to oxidative stress76. In a chronic ocular hypertension rat model, H2S is demonstrated to attenuate RGC apoptosis through balancing mitochondrial function, suppressing glial activation and downregulating the autophagy process78. Intracameral injection of NaHS to rats bearing glaucoma prohibits the loss of RGCs through recovering the levels of H2S in retina79. A long time release of H2S from GYY4137 combined with the in situ gel forming PLGA-based system, which lasts up to 72 h, has pointed to a great potential application in treating glaucoma80.(Fig. 2)
H2S donors lower IOP by the reduction of the trabecular meshwork cell volume, the promotion of AH outflow, the relaxation of iris smooth muscles and the decrease of norepinephrine release. H2S can relax PCAs to modulate the OBF, so that alleviates the glaucomatous damage induced by ischemia. It also attenuates cellular damage induced by oxidants and inhibits neuron apoptosis to protect RGCs
H2S and diabetic retinopathy (DR)
Reduction of the effects of advanced glycation end products (AGEs) in DR
High glucose condition gives rise to the non-enzymatic condensation reaction between glucose and the amino terminus of protein, leads to the accumulation of AGEs’ macromolecule, which has close relationship with the occurrence of DR81,82. AGEs can crosslink intracellular proteins to disturb their functions, and interfere normal metabolic pathways such as ATP production. AGEs destroys the inner blood–retinal barrier (BRB) in eye with subsequent oxidative stress reactions and inflammation83,84.
H2S promotes galactose metabolism to reduce AGEs generation in neuronal cells and prohibit excessive oxidative stress85. Mechanistically, H2S reduces ROS production and lipid peroxidation, while enhancing the expression of superoxide dismutase (SOD) and glutathione peroxidase (GPX), two endogenous antioxidant enzymes86. In addition, H2S could reverse high glucose-induced increase in the expression of aldehyde oxidase 1 (AOX-1) and decrease in glutathione synthetase (GSS) level, ultimately to antagonize the AGEs-induced oxidative stress in cells85.
Inhibition of oxidative stress and inflammation
Although the toxicity of H2S accounts for the pathogenesis of multiple diseases, H2S possesses versatile anti-inflammatory effects in vivo or vitro.
High glucose levels disturb the electron transfer process of the cellular mitochondrial respiratory chain in diabetic patients, so that oxygen free radical O− and superoxide can be easily generated87. Excessive O− converts NO into ONOO−, which can irreversibly bind to cytochrome C and impair mitochondrial functions. In DR animal models, the enhanced level of intracellular oxygen species and its associated excessive lipid peroxidation can be suppressed by H2S73,88. One property of H2S in anti-inflammation is to scavenge the pro-inflammatory oxidants, such as ONOO−, HOCl, superoxide and hydrogen peroxide71,89. Besides, the pro-inflammatory response can be shifted to anti-inflammation by H2S donors, as demonstrated to decrease the levels of TNF-α, IL-8 and IFN-γ, while increasing the levels of cyclooxygenase (COX)-2 and eicosanoids90. Similarly, GYY4137 has been reported to inhibit LPS-induced production of inflammatory mediators by macrophages, and to upregulate the release of anti-inflammatory cytokine, IL-1091. Such regulation on inflammatory cytokine production can be attributed to the suppressive function of H2S on NF-κB activation91,92.
The animal models of DR have showed that hyperglycemia-induced leukostasis is related with cell apoptosis and retinal capillary occlusion93. The effect of resolving inflammation by H2S relies on its role in mediating macrophage phagocytosis94 and promoting the granulocytes survival through inhibition of p38 phosphorylation and caspase-3 cleavage95. It downregulates the expression of MPO in neutrophils, thereby alleviating some of their toxic actions96. Moreover, increased retinal expression of intercellular adhesion molecule-1 (ICAM-1) and leukocyte adhesion in vessels are observed in DR animal models93, but H2S could downregulate ICAM-1 expression in vascular endothelium under high glucose conditions97. The adhesion molecules, including lymphocyte function-associated antigen, P-selectin and ICAM-1, are indispensable in instructing immune cells to transmigrate across inflamed capillaries. Blockade of H2S synthetase abolishes the alleviation of inflammation, with increased adherence of leukocytes to vascular endothelium and their transmigration98,99.
Investigations on the regulation of H2S on myocardium in type 1 diabetic rat model has revealed that H2S interferes with the inducible NOS (iNOS)/NO system, inhibits iNOS activity and its catabolite mediated oxidative stress100. However, the anti-inflammatory function by H2S is not always achieved. In low dose, H2S donor inhibits the inflammatory response, while high doses of H2S donor achieves controversial results. Therefore, dosage is a switch to control the biphasic regulation of H2S donor on inflammation101, and the generation of H2S can be augmented by the appearance of inflammation102. The feedback mechanism of H2S in controlling the progression of inflammatory responses in DR remains unclear.
Protective effect on retinal neurons
During the pathological process of DR, neuron damage usually occurs earlier and accumulates into visible retinal vascular lesions103. ACS67, a H2S donor, can be used to prevent RGC apoptosis and reactive gliosis in Muller cells after ischemia-reperfusion or exposure to oxidative stress104. Also, administration of H2S donors recovers the expression of brain-derived neurotrophic factor (BDNF) and retinal synaptic vesicle protein in streptozotocin (STZ)-induced diabetic rats, indicating that H2S might block neuronal degeneration of retinal in diabetic patients105. The neuroprotective effect of H2S in retina is also related to its regulation on the intracellular GSH content104.
Multiple effects on retinal blood vessels
Dual role of BRB stability
The dysfunction of BRB is a primary cause of retinal vascular lesions during DR pathogenesis. In DR development, retinal ischemia and hypoxia stimulate the expression of hypoxia inducible factor (HIF-1α) and trigger subsequent vascular endothelial growth factor (VEGF) signaling activation. The HIF-1α-VEGF-VEGFR2 signaling pathway is responsible for diabetes-induced BRB dysfunction and excessive angiogenesis106. In vivo experiments have shown that the reduced BRB permeability and decreased acellular capillaries in retinas of STZ-induced diabetic rats after exogenous H2S treatment is accompanied by the reduction in VEGF content of vitreous and gene expression of VEGFR2, HIF-1α, as well as with increased expression of occludin105. Exogenous H2S administration is found to inhibit excessive deposition of laminin and collagen IVα3, in order to maintain the vascular integrity in the retinas of diabetic rats107. On the other hand, VEGF in intraocular tissues can stimulate endothelial cells to produce and release H2S108. At the onset of diabetes, H2S served as a protective factor against oxidative stress or nitrosative stress in the retina and vitreous humor, and it seems like H2S has a protective role on BRB in hyperglycemic condition. However, along with the progression of proliferative diabetic retinopathy (PDR), H2S may enhance the effect of VEGF on vascular endothelial cells, as well as the angiogenesis process108,109,110. Investigations on the level of H2S in the vitreous and plasma of PDR patients have revealed a much higher expression, indicating the potential effects of H2S in the pathogenesis of PDR111. As one of the main source to produce H2S in retina, 3-MST in hyperglycemic cells fail to convert 3-MP to H2S when the extracellular glucose concentration is elevated, and thus lost the ability of stimulating angiogenesis or cell proliferation, but the proangiogenic effect by exogenous H2S is not attenuated by hyperglycemia112. Moreover, the H2S-generating enzymes/H2S contributes to retinal neovascularization in ischemia-induced retinopathy113. These facts indicate that H2S may deteriorate retinal hemorrhage during the late stages of PDR.
Antithrombotic effect
Besides inflammation and apoptosis, platelet adhesion is also involved in diabetes-induced retinal endothelial dysfunction93. The platelet adhesion to the injured diabetic endothelium takes part in ischemia and inflammation, both coagulation and fibrinolytic cascades in the vitreous are identified in DR114. Blood platelets tend to adhere to the vascular endothelium of DR rather than normal vessels115, which is involved in retinal capillaries occlusion and microvascular damage. H2S plays a potential role in reducing platelet aggregation, cell adhesion, and coagulantion116,117,118,119, it exerts antithrombotic effect through upregulation of NO synthesis, hydrolysis of disulfide bonds and the reduction of the calcium concentration in platelets119,120,121.
Modulation of the retinal blood flow
The altered retinal circulation of the diabetes is well documented, diabetic mice demonstrates reduced density of flowing deep vessels122. There may be both increased and decreased retinal blood flow in diabetic patients compared with healthy people, while no significant difference is observed in OBF between patients of nonproliferative DR and PDR123. Considering the possibility of ischemia and hypoxia induced by abnormal blood supply and vascular dysfunction, we notice that H2S has multiple effects on vessels. The application of H2S donors could protect blood vessels, regulate blood pressure and alleviate the inflammatory reactions in the vascular system124. H2S exhibits the dual vascular effects of vasoconstriction and vasodilation depends on the vascular district, the endothelium conditions, the H2S concentration and the method of precontraction125. Different from the increased cAMP production induced by H2S in brain cells59, H2S negatively modulates β-adrenoceptor function via suppressing the adenylyl cyclase activity in cardiac myocytes126. The adenyl cyclase/cAMP pathway is involved in H2S induced vasoconstriction127, but on the other hand, H2S can instruct vascular smooth muscle cells against excessive vascular contraction via affecting KATP9. Moreover, H2S alleviates the contraction of vascular smooth muscle by reducing the concentration of intracellular calcium through acting on inositol 1,4,5-triphosphate receptor128. While its variable effects on vasculature were still being discussed, the increased cGMP level due to the PDE inhibition, the affected NO/cGMP pathway with activated endothelial nitric oxide synthase (eNOS) and COX-derived metabolic byproducts are all required for H2S-induced vasodilation129,130. In addition, the vasodilation induced by H2S is related to the promotion of prostaglandin generation131, angiotensin-converting enzyme inhibition102, as well as modulating the viability of anion exchangers to control intra-cellular pH value132. All these findings imply that H2S contributes to regulating retinal blood flow and is involved in the DR pathogenesis. (Fig. 3)
H2S promotes galactose metabolism to reduce AGE generation in neuronal cells and antagonizes high glucose-induced oxidative stress and inflammation, as well as protects retinal neurons in diabetic patients. Although H2S can relief the BRB permeability of DR and exert an antithrombotic effect, it shows dual vascular effects on retinal vessels to modulate the retinal blood flow and participates in angiogenesis process
H2S and retinal degeneration
Modulation and protection of retinal neurons
Several retinal degenerative diseases, such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD), are associated with aberrant function of retinal pigment epithelium (RPE) and photoreceptor cells, which are crucial to maintain accurate visual sense133.
One of the RPE features is the apical and basal membranes, where the apical parts envelop photoreceptor cell outer segment (POS) to remove and degrade them through phagocytosis134. With a circadian rhythm, the phagocytosis of POS distal tips is always triggered by light135. Disturbance of RPE phagocytic function leads to POS accumulation and inevitable photoreceptor degeneration. The deficiency of CBS activity and the accumulation of homocysteine in the retina may lead to abnormal RPE structure and functions, bringing about the development of AMD-like features136.
Another feature of RPE cells is melanogenesis for absorbing excess light and protect photoreceptors. Melanin dispersion toward the apical microvilli of the RPE correlates positively with the intracellular level of cAMP, while light suppresses cAMP synthesis in retina of mice137. It has been shown that cAMP stimulates melatonin synthesis138 and elevated cAMP levels with its signaling system influences RPE migration139. Increased cAMP in the subretinal space can lead to entry of cAMP into RPE cells via organic anion transporters with consequent triggering of dark-specific physiological responses, the nonderivatized cAMP can activate pigment granule aggregation in isolated RPE sheets140. It is reported that H2S donors and its substrate could produce a time and dose-dependent increase in cAMP concentrations in rat RPE cells141, the process of which involves KATP channels and the enzymes of CSE and CBS141.
The metabolic cascade of photoreceptor signal transduction is mediated by cGMP that synthesized by guanylyl cyclases in retinal neurons. The response is triggered when photopigments absorb light, with subsequent degradation of cGMP by PDE142. Activities of cAMP-hydrolyzing and cGMP-hydrolyzing have been detected in homogenates of cultured pigment epithelia from rats143. It is reported that cGMP stimulates the absorption of subretinal fluid by activating the RPE cell pump144, which is consistent with the fact of decreased cGMP concentration in the retinal detachment cases142. As mentioned above, H2S participates in the inhibition of PDE activity and induction of cyclic nucleotides, and at least three forms of PDEs are present in human RPE cells145, we infer that the cumulative cAMP or cGMP instructed by H2S may help maintain physiological functions of RPE and photoreceptors.
Administration of H2S contributes to protecting retinal neurons from light-induced degeneration26. Chronic sustained light-induced damages in the macular area cause degeneration of RPE and photoreceptor cells. Long-term excessive light exposure can induce damage or death of photoreceptor cells by oxidative stress and intracellular calcium overload146. Calcium in relatively low level can activate the 3MST/CAT enzymes to produce H2S. In turn, H2S can prevent Ca2+ influx in the photoreceptor cells by activating V-ATPase in horizontal cells and maintain the balance of intracellular calcium, so that H2S protects photoreceptor cells from retinal cell apoptosis and oxidative stress26. However, the regulation of Ca2+ and the cytoprotective effect of endogenous H2S may fail when photoreceptor cells are under excessive light exposure.
Potential in stem cell transplantation therapy
The RPE cells can modulate photoreceptor differentiation and retinal progenitor cells, which may play a role in the regulation of the retinal stem cell niche147. Transplantation of stem cell-derived RPE is proven to be effective in reversing retinal degeneration such as AMD148,149. MSCs are multipotent stem cells with self-renewal abilities, immunoregulatory functions and multiple lineage differentiation potentials. In vitro expanded MSCs have been widely applied to treat many tissue injury, such as myocardial infarction150, skin wound151, organ transplantation152, autoimmune diseases153, and retina injuries154. MSCs express CBS and CSE, and produce H2S155, with a positive feedback on the proliferation and survival of MCSs156. Studies have found that increased endogenous H2S level can block the hypoxia and serum deprivation-induced MSC apoptosis157, both the ERKs signaling pathways and the Akt signaling pathway are involved in the promotion of H2S on stem cell proliferation158,159. NaHS can prolong the survival of bone marrow mesenchymal stem cells (BMMSCs) and enhance their therapeutic effects for ischemic injury, also can improve the blood vessel integrity and prompt angiogenesis, with the upregulation of BDNF and VEGF expression160. In its regulation on stem cell differentiation, H2S is likely to affect neurogenesis by directly regulating Ca2+ channels161, to initiate endothelial progenitor cell function and to enhance the angiogenesis process of wound sites in type 2 diabetic patients162.
Also, H2S is featured as one of the potential molecule for immunoregulation by MSCs. Deficiency of H2S attenuates the immunosuppressive function of MSCs on colitis in vivo, while supplementation of NaHS can restore the impaired therapeutic effects163. By the way, clinical H2S treatment is expected to improve long-term allograft survival in conjunction with immunosuppression for its positive effects on promoting organ survival against cold ischemia reperfusion injury164. Considering that NaHS pretreatment can enhance stem cells proliferation, promote the survival of therapeutically used stem cells and tissue cells via increased antioxidant defense165, H2S may be useful for the regeneration of retinal photoreceptors and RPE cells via transplantation strategies (Fig. 4).
H2S and its productive enzymes are involved in the phagocytosis of POS and melanogenesis in RPE cells, as well as in photoreceptor signal transduction. Long-term excessive light exposure induces photoreceptor cell damage which is related to intracellular calcium overload. When the retinal photoreceptor cells are exposed under high intensity illumination, the cGMP-gated ion channels in membrane are shut down, with a cascade activity resulted in a relative low level of intracellular calcium. Such status facilitates H2S generation catalyzed by 3MST/CAT enzymes, subsequently suppress Ca2+ influx by activating V-ATPase. Exogenous H2S reduces the number of apoptotic retinal neurons after excessive light irradiation. Another approach to treating retinal degeneration disease is the stem cell-derived RPE-based therapy. Studies have found that H2S affects the immunoregulatory function of MSCs, and can enhance the proliferation and survival of the stem cells, thus improve their ability in tissue repair
Perspectives
Investigations in the past decade have provided new insights into the function of H2S during tissue damage and repair. In addition to its toxic effects, H2S is found to reduce intraocular pressure, inhibit inflammation and oxidative stress, promote stem cell-based regeneration, and restore the retinal microcirculation homeostasis. However, the exact therapeutic and pathological concentration of H2S remains elusive. Recently, novel H2S releasing drugs such as ATB-346 and ATB-352, have shown the efficacy in treating digestive diseases, with the promising application potential in various eye diseases. Due to the complexity of the BRB and the special anatomical structures of the eyes, the administrative routes of H2S should be carefully considered. Further investigation in this exciting field is expected to provide detailed information for better understanding the function of H2S in different types of eye diseases, and to design more effective and safe approaches for H2S application in clinical settings.
References
Olson, K. R., Donald, J. A., Dombkowski, R. A. & Perry, S. F. Evolutionary and comparative aspects of nitric oxide, carbon monoxide and hydrogen sulfide. Respir. Physiol. Neurobiol. 184, 117–129 (2012).
Olson, K. R., DeLeon, E. R. & Liu, F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide 41, 11–26 (2014).
Olas, B. Hydrogen sulfide in hemostasis: friend or foe? Chem.-Biol. Interact. 217, 49 (2014).
Olson, K. R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim. Et. Biophys. Acta Bioenerg. 1787, 856–863 (2009).
Hogg, P. J. Contribution of allosteric disulfide bonds to regulation of hemostasis. J. Thromb. Haemost. 7, 13–16 (2009).
Zhong, G., Chen, F., Cheng, Y., Tang, C. & Du, J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 21, 1879 (2003).
Olas, B. Hydrogen sulfide in signaling pathways. Clin. Chim. Acta 439, 212–218 (2015).
Saito, J. et al. Sputum hydrogen sulfide as a novel biomarker of obstructive neutrophilic asthma. J. Allergy Clin. Immunol. 131, 232 (2013).
Kabil, O., Motl, N. & Banerjee, R. H2S and its role in redox signaling. Biochim Biophys. Acta 1844, 1355–1366 (2014).
Reiffenstein, R. J., Hulbert, W. C. & Roth, S. H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 32, 109 (1992).
Szabo, C. A timeline of hydrogen sulfide (H2S) research: from environmental toxin to biological mediator. Biochem Pharmacol. 149, 5–19 (2018).
Mustafa, A. K. et al. H2S signals through protein S-sulfhydration. Sci. Signal. 2, ra72 (2009).
Filipovic, M. R. Persulfidation (S-sulfhydration) and H2S. Handb. Exp. Pharmacol. 230, 29–59 (2015).
Kimura, H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 63, 492–497 (2013).
Yang, G. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322, 587 (2009).
Gemici, B. & Wallace, J. L. Anti-inflammatory and cytoprotective properties of hydrogen sulfide. Methods Enzymol. 555, 169–193 (2015).
Kulkarni, M. et al. Endogenous production of hydrogen sulfide in isolated bovine eye. Neurochem. Res. 36, 1540–1545 (2011).
Hughes, M. N. & Centelles MNMoore, K. P. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic. Biol. Med. 47, 1346–1353 (2009).
Tanizawa, K. Production of H2S by 3-mercaptopyruvate sulphurtransferase. J. Biochem. 149, 357 (2011).
Abe, K. & Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 16, 1066–1071 (1996).
Pan, Y. et al. Hydrogen sulfide (H 2 S)/cystathionine γ-lyase (CSE) pathway contributes to the proliferation of hepatoma cells. Mutat. Res./Fundam. Mol. Mech. Mutagen. S. 763–764, 10–18 (2014).
Lefer, D. J. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc. Natl Acad. Sci. USA 104, 17907–17908 (2007).
Han, S. J. et al. Hydrogen sulfide-producing cystathionine γ-lyase is critical in the progression of kidney fibrosis. Free Radic. Biol. & Med. 112, 423 (2017).
Persa, C., Osmotherly, K., Chen, C. W., Moon, S. & Louabcd, M. F. The distribution of cystathionine β-synthase (CBS) in the eye: Implication of the presence of a trans-sulfuration pathway for oxidative stress defense. Exp. Eye Res. 83, 817–823 (2006).
Winnie, W. et al. Comparative localization of cystathionine beta-synthase and cystathionine gamma-lyase in retina: differences between amphibians and mammals. J. Comp. Neurol. 505, 158–165 (2010).
Mikami, Y. et al. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2 + influx. J. Biol. Chem. 286, 39379 (2011).
Shibuya, N. et al. P33 A novel pathway for the production of hydrogen sulfide from d -cysteine in mammalian cells. Nitric Oxide 39, 1366 (2014).
Picker, J. D. & Levy, H. L. Homocystinuria Caused by Cystathionine Beta-synthase Deficiency. (University of Washington, Seattle, 2010).
Yu, M. et al. Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp. Eye Res. 96, 124–131 (2012).
Ganapathy, P. S. et al. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-β-synthase mutant mouse. Invest Ophthalmol. Vis. Sci. 50, 4460–4470 (2009).
Tawfik, A. et al. Alterations of retinal vasculature in cystathionine-β-synthase heterozygous mice: a model of mild to moderate hyperhomocysteinemia. Am. J. Pathol. 184, 2573–2585 (2014).
Maggio, F. Glaucomas. Top. Companion Anim. Med. 30, 86–96 (2015).
Mantravadi, A. V. & Vadhar, N. Glaucoma. Prim. Care Clin. Off. Pract. 42, 437–449 (2015).
Neufeld, A. H., Dueker, D. K., Vegge, T. & Sears, M. L. Adenosine 3’,5’-monophosphate increases the outflow of aqueous humor from the rabbit eye. Invest. Ophthalmol. 14, 40 (1975).
Robinson, J. et al. Effects of hydrogen sulfide-releasing compounds on aqueous humor outflow facility in porcine ocular anterior segments, ex vivo. J. Ocul. Pharmacol. 33, 2 (2017).
Módis, K. et al. OP21 Role of phosphodiesterase inhibition and modulation of mitochondrial cAMP levels in the bioenergetic effect of hydrogen sulfide in isolated mitochondria. Nitric Oxide 31, S28–S28 (2013).
Bucolo, C. & Drago, F. Carbon monoxide and the eye: implications for glaucoma therapy. Pharmacol. Ther. 130, 191–201 (2011).
Perrino, E. et al. New prostaglandin derivative for glaucoma treatment. Bioorg. Med. Chem. Lett. 19, 1639–1642 (2009).
Salvi, A. et al. Effect of hydrogen sulfide donors on intraocular pressure in rabbits. J. Ocular Pharmacol. Therapeut. 32, 371–5 (2016).
Monjok, E. M. et al. Inhibitory action of hydrogen sulfide on muscarinic receptor-induced contraction of isolated porcine irides. Exp. Eye Res. 87, 612 (2008).
Zhan, G. L. et al. Time dependent effects of sympathetic denervation on aqueous humor dynamics and choroidal blood flow in rabbits. Curr. Eye Res. 25, 99 (2002).
Yoshitomi, T., Horio, B. & Gregory, D. S. Changes in aqueous norepinephrine and cyclic adenosine monophosphate during the circadian cycle in rabbits. Invest Ophthalmol. Vis. Sci. 32, 1609–1613 (1991).
Kulkarni, K. H. et al. Effect of hydrogen sulfide on sympathetic neurotransmission and catecholamine levels in isolated porcine iris-ciliary body. Neurochem. Res. 34, 400 (2009).
Flammer, J. The impact of ocular blood flow in glaucoma. Progress. Retin. Eye Res. 21, 359–393 (2002).
Salomone, S. et al. Regulation of vascular tone in rabbit ophthalmic artery: cross talk of endogenous and exogenous gas mediators. Biochem. Pharmacol. 92, 661–668 (2014).
Madhura, K. C. et al. Inhibitory action of novel hydrogen sulfide donors on bovine isolated posterior ciliary arteries. Exp. Eye Res. 134, 73–79 (2015).
Chitnis, M. K. et al. Pharmacological actions of the slow release hydrogen sulfide donor GYY4137 on phenylephrine-induced tone in isolated bovine ciliary artery. Exp. Eye Res. 116, 350–354 (2013).
Denoyer, A. et al. [Retinal and trabecular degeneration in glaucoma: new insights into pathogenesis and treatment]. J. Fr. D. Ophtalmol. 38, 347 (2015).
Goyal, A., Srivastava, A., Sihota, R. & Kaur, J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr. Eye Res. 39, 823 (2014).
Osborne, N. N. & del Olmo-Aguado, S. Maintenance of retinal ganglion cell mitochondrial functions as a neuroprotective strategy in glaucoma. Curr. Opin. Pharmacol. 13, 16–22 (2013).
W.-K., J. et al. Increased mitochondrial fission and volume density by blocking glutamate excitotoxicity protect glaucomatous optic nerve head astrocytes. Glia 63, 736–753 (2015).
Maher, P. & Hanneken, A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Invest Ophthalmol. Vis. Sci. 46, 749 (2005).
Kamat, P. K., Kalani, A. & Tyagi, N. Role of hydrogen sulfide in brain synaptic remodeling. Methods Enzymol. 555, 207–229 (2015).
White, B. J. O., Smith, P. A. & Dunn, W. R. Hydrogen sulphide–mediated vasodilatation involves the release of neurotransmitters from sensory nerves in pressurized mesenteric small arteries isolated from rats. Br. J. Pharmacol. 168, 785–793 (2013).
Nagai, Y., Tsugane, M., Oka, J. & Kimura, H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 18, 557–559 (2004).
Salvi, A. et al. Pharmacological actions of hydrogen sulfide donors on sympathetic neurotransmission in the bovine anterior uvea, in vitro. Neurochem. Res. 41, 1–9 (2016).
Bankhele, P. et al. Comparative effects of hydrogen sulfide-releasing compounds on [3H]D-aspartate release from bovine isolated retinae. Neurochem. Res. 43, 692–701 (2018).
Kamat, P. K., Kalani, A., Tyagi, S. C. & Tyagi, N. Hydrogen sulfide epigenetically attenuates homocysteine-induced mitochondrial toxicity mediated through NMDA receptor in mouse brain endothelial (bEnd3) cells. J. Cell Physiol. 230, 378–394 (2015).
Kimura, H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys. Res Commun. 267, 129–133 (2000).
Marutani, E. et al. P47 A novel hydrogen sulfide-releasing NMDA receptor antagonist prevents ischemic neuronal death. J. Biol. Chem. 27, S33–S33 (2012).
Arima, K. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 293, 1485–1488 (2002).
Paul, B. D. et al. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington’s disease. Nature 509, 96–100 (2016).
Vandiver, M. S. et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 4, 1626 (2013).
Davoli, A. et al. Evidence of hydrogen sulfide involvement in amyotrophic lateral sclerosis. Ann. Neurol. 77, 697–709 (2015).
Kida, K. et al. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson’s disease. Antioxid. Redox Signal. 15, 343–352 (2011).
Shefa, U., Kim, M. S., Jeong, N. Y. & Jung, J. Antioxidant and cell-signaling functions of hydrogen sulfide in the central nervous system. Oxid. Med. Cell. Longev. 2018, 1–17 (2018).
Paul, B. D. & Snyder, S. H. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem. Pharmacol. 149, 101–109 (2017).
Kimura, Y., Goto, Y. & Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 12, 1 (2010).
Kimura, Y., Dargusch, R., Schubert, D. & Kimura, H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid. Redox Signal 8, 661–670 (2006).
Kumar, M. & Sandhir, R. Neuroprotective effect of hydrogen sulfide in hyperhomocysteinemia is mediated through antioxidant action involving Nrf2. Neuromol. Med. 20, 475–490 (2018).
Whiteman, M. et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys. Res. Commun. 326, 794–798 (2005).
Hu, L. F., Lu, M., Wu, Z. Y., Wong, P. T. & Bian, J. S. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol. Pharmacol. 75, 27–34 (2009).
Kimura, H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 41, 4–10 (2014).
Kimura, Y. & Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 18, 1165 (2004).
Chan, J. Y. & Kwong, M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys. Acta 1517, 19–26 (2000).
Majid, A. S. A., Majid, A. M. S. A., Yin, Z. Q. & Ji, D. Slow regulated release of H2S inhibits oxidative stress induced cell death by influencing certain key signaling molecules. Neurochem. Res. 38, 1375–1393 (2013).
Osborne, N. N., Ji, D., Majid, A. S. A., Del Soldata, P. & Sparatore, A. Glutamate oxidative injury to RGC-5 cells in culture is necrostatin sensitive and blunted by a hydrogen sulfide (H2S)-releasing derivative of aspirin (ACS14). Neurochem. Int. 60, 365–378 (2012).
Huang, S. et al. Hydrogen sulfide supplement attenuates the apoptosis of retinal ganglion cells in experimental glaucoma. Exp. Eye Res. 168, 33–48 (2018).
Huang, S. et al. Relevant variations and neuroprotecive effect of hydrogen sulfide in a rat glaucoma model. Neuroscience 341, 27–41 (2017).
Patil, A., Singh, S., Opere, C. & Dash, A. Sustained-release delivery system of a slow hydrogen sulfide donor, GYY 4137, for potential application in glaucoma. AAPS PharmSciTech 18, 2291–2302 (2017).
Yang, L. et al. Association of the receptor for advanced glycation end products gene polymorphisms and circulating RAGE levels with diabetic retinopathy in the Chinese population. J. Diabetes Res. 2013, 1–8 (2013).
Padayatti, P. S., Jiang, C., Glomb, M. A., Uchida, K. & Nagaraj, R. H. High concentrations of glucose induce synthesis of argpyrimidine in retinal endothelial cells. Curr. Eye Res. 23, 106 (2001).
Kandarakis, S. A., Piperi, C., Topouzis, F. & Papavassiliou, A. G. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Progress. Retin. Eye Res. 42, 85–102 (2014).
Hirata, C. et al. Advanced glycation end products induce expression of vascular endothelial growth factor by retinal müller cells. Biochem. Biophys. Res. Commun. 236, 712–715 (1997).
Liu, Y.-Y., Nagpure, B. V., Wong, P. T. H. & Bian, J.-S. Hydrogen sulfide protects SH-SY5Y neuronal cells against d-galactose induced cell injury by suppression of advanced glycation end products formation and oxidative stress. Neurochem. Int. 62, 603–609 (2013).
Usui, S. et al. Overexpression of SOD in retina: need for increase in H2O2-detoxifying enzyme in same cellular compartment. Free Radical Biol. Med. 51, 1347 (2011).
Brownlee, M. The pathobiology of diabetic complications. Diabetes 54, 1615–1625 (2005).
Geng, B. et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. & Biophys. Res. Commun. 318, 756–763 (2004).
Muzaffar, S. et al. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J. Vasc. Res. 45, 521–528 (2008).
Gemici, B. et al. H2S-releasing drugs: anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide 46, 25–31 (2015).
Whiteman, M. et al. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 12, 1147–1154 (2010).
Ling, L. et al. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 42, 706 (2007).
Miyamoto, K. et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl Acad. Sci. USA 96, 10836–10841 (1999).
Dufton, N., Natividad, J., Verdu, E. F. & Wallace, J. L. Hydrogen sulfide and resolution of acute inflammation: a comparative study utilizing a novel fluorescent probe. Sci. Rep. 2, 499 (2012).
Rinaldi, L. et al. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab. Investig. 86, 391 (2006).
Pálinkás, Z. et al. Interactions of hydrogen sulfide with myeloperoxidase. Br. J. Pharmacol. 172, 1516–1532 (2015).
Guan, Q. et al. Hydrogen sulfide suppresses high glucose-induced expression of intercellular adhesion molecule-1 (ICAM-1) in endothelial cells. J. Cardiovasc. Pharmacol. 62, 278–284 (2013).
Zanardo, R. C. O. et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 20, 2118–2120 (2006).
Fiorucci, S. et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 129, 1210–1224 (2005).
Yang, R., Jia, Q., Liu, X. F., Wang, Y. Y. & Ma, S. F. Effects of hydrogen sulfide on inducible nitric oxide synthase activity and expression of cardiomyocytes in diabetic rats. Mol. Med. Rep. 16, 5277–5284 (2017).
Lo Faro, M. L., Fox, B., Whatmore, J. L., Winyard, P. G. & Whiteman, M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide Biol. Chem. 41, 38 (2014).
Oh, G. S. et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-κB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 41, 106–119 (2006).
Lieth, E., Gardner, T. W., Barber, A. J. & Antonetti, D. A. Retinal neurodegeneration: early pathology in diabetes. Clin. Exp. Ophthalmol. 28, 3–8 (2000).
Osborne, N. N. et al. ACS67, a Hydrogen Sulfide–Releasing Derivative of Latanoprost Acid, Attenuates Retinal Ischemia and Oxidative Stress to RGC-5 Cells in Culture. Invest. Opthalmology Vis. Sci. 51, 284 (2010).
Si, Y.-F. et al. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br. J. Pharmacol. 169, 619–631 (2013).
Qaum, T. et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest. Ophthalmol. Vis. Sci. 42, 2408 (2001).
Oshitari, T. et al. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes 55, 86 (2006).
Papapetropoulos, A. et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl Acad. Sci. USA 106, 21972 (2009).
Csaba Szabó, A. P. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br. J. Pharmacol. 164, 853–865 (2011).
Cai, W. J. et al. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 76, 29–40 (2007).
Ran, R. et al. Elevated hydrogen sulfide levels in vitreous body and plasma in patients with proliferative diabetic retinopathy. Retina 34, 2003–2009 (2014).
Coletta, C. et al. Regulation of Vascular Tone, Angiogenesis and Cellular Bioenergetics by the 3-Mercaptopyruvate Sulfurtransferase/H2S Pathway: Functional Impairment by Hyperglycemia and Restoration by DL-α-Lipoic Acid. Mol. Med. 21, 1–14 (2015).
Gersztenkorn, D. et al. Hydrogen sulfide contributes to retinal neovascularization in ischemia-induced retinopathy. Invest Ophthalmol. Vis. Sci. 57, 3002–3009 (2016).
Nivetha, M., Tuna, Ü. & Feener, E. P. Thrombosis and hemorrhage in diabetic retinopathy: a perspective from an inflammatory standpoint. Semin. Thromb. & Hemost. 41, 659–664 (2015).
Vinik, A. I., Erbas, T., Park, T. S., Nolan, R. & Pittenger, G. L. Platelet dysfunction in type 2 diabetes. Diabetes Care 24, 1476–1485 (2001).
Morel, A., Malinowska, J. & Olas, B. Antioxidative properties of hydrogen sulfide may involve in its antiadhesive action on blood platelets. Clin. Biochem. 45, 1678–1682 (2012).
Morel, A., Malinowska, J. & Olas, B. Hydrogen sulfide changes adhesive properties of fibrinogen and collagen in vitro. Platelets 25, 147 (2014).
Nishikawa, H. et al. Inhibition by hydrogen sulfide of rabbit platelet aggregation and calcium mobilization. Biol. Pharm. Bull. 36, 1278–1282 (2013).
Grambow, E. et al. Effect of the hydrogen sulfide donor GYY4137 on platelet activation and microvascular thrombus formation in mice. Platelets 25, 166 (2014).
Lukas, K., Eberhard, G., Fabian, M. G. & Heiko, S. & Brigitte, V. The anti-thrombotic effect of hydrogen sulfide is partly mediated by an upregulation of nitric oxide synthases. Thromb. Res. 132, 112–117 (2013).
Wachowicz, B., Olas, B., Zbikowska, H. M. & Buczyński, A. Generation of reactive oxygen species in blood platelets. Platelets 13, 175 (2002).
Uehara, H. et al. Detection of microvascular retinal changes in type I diabetic mice with optical coherence tomography angiography. Exp. Eye Res. 178, 91–98 (2019).
Ozkiris, A., Erkiliç, K., Koç, A. & Mistik, S. Effect of atorvastatin on ocular blood flow velocities in patients with diabetic retinopathy. Br. J. Ophthalmol. 91, 69–73 (2007).
Polhemus, D. J. & Lefer, D. J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 114, 730–737 (2014).
D’Emmanuele, dV. B. R. et al. Hydrogen sulfide-induced dual vascular effect involves arachidonic acid cascade in rat mesenteric arterial bed. J. Pharmacol. Exp. Ther. 337, 59 (2011).
Qian Chen, Y., Ting-Ting, P., Li-Fang, H. & Jin-Song, B. Negative regulation of beta-adrenergic function by hydrogen sulphide in the rat hearts. J. Mol. Cell. Cardiol. 44, 701–710 (2008).
Lim, J. J., Liu, Y. H., Khin, E. S. & Bian, J. S. Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 295, C1261 (2008).
Castro-Piedras, I. & Perez-Zoghbi, J. F. Hydrogen sulphide inhibits Ca 2 + release through InsP 3 receptors and relaxes airway smooth muscle. J. Physiol. 591, 5999 (2013).
Kutz, J. L., Greaney, J. L., Santhanam, L. & Alexander, L. M. Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature. J. Physiol. 593, 2121–2129 (2015).
Sofia-Iris, B. et al. Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc. Res. 106, 432–442 (2015).
Koenitzer, J. R. et al. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 292, H1953 (2007).
Lee, S. W., Cheng, Y., Moore, P. K. & Bian, J. S. Hydrogen sulphide regulates intracellular pH in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 358, 1142–1147 (2007).
Olaf, S. The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845–881 (2005).
Young, R. W. & Bok, D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 42, 392–403 (1969).
Bertolotti, E., Neri, A., Camparini, M., Macaluso, C. & Marigo, V. Stem cells as source for retinal pigment epithelium transplantation. Progress. Retin. Eye Res. 42, 130–144 (2014).
Ibrahim, A. S. et al. Hyperhomocysteinemia disrupts retinal pigment epithelial structure and function with features of age-related macular degeneration. Oncotarget 7, 8532–8545 (2016).
Sarangarajan, R. & Apte, S. P. Melanization and phagocytosis: implications for age related macular degeneration. Mol. Vision. 11, 482–490 (2005).
Iuvone, P. M. & Besharse, J. C. Regulation of indoleamine N-Acetyltransferase activity in the retina: Effects of light and dark, protein synthesis inhibitors and cyclic nucleotide analogs. Brain Res. 273, 111–119 (1983).
Smith-Thomas, L. C. et al. Influence of pigment content, intracellular calcium and cyclic AMP on the ability of human retinal pigment epithelial cells to contract collagen gels. Curr. Eye Res. 21, 518–529 (2000).
García, D. M. & Burnside, B. Suppression of cAMP-induced pigment granule aggregation in RPE by organic anion transport inhibitors. Invest. Ophthalmol. Vis. Sci. 35, 178 (1994).
Njie-Mbyea, Y. F., Opere, C. A. & Ohia, S. E. Mechanism of action of hydrogen sulfide on cyclic AMP formation in rat retinal pigment epithelial cells. Exp. Eye Res. 98, 16–22 (2012).
La Heij, E. C. et al. Decreased levels of cGMP in vitreous and subretinal fluid from eyes with retinal detachment. Br. J. Ophthalmol. 87, 1409 (2003).
Kurtz, M. J., Edwards, R. B. & Schmidt, S. Y. Cyclic nucleotide phosphodiesterases in cultured normal and RCS rat pigment epithelium: kinetics of cyclic AMP and cyclic GMP hydrolysis. Exp. Eye Res. 45, 67–75 (1987).
Marmor, M. F. & Negi, A. Pharmacologic modification of subretinal fluid absorption in the rabbit eye. Arch. Ophthalmol. 104, 1674 (1986).
Diederen, R., Kijlstra, A., Hendrikse, F. & De-Vente, J. Selective blockade of phosphodiesterase types 2, 5 and 9 results in cyclic 3'5’ guanosine monophosphate accumulation in retinal pigment epithelium cells. Br. J. Ophthalmol. 91, 379–384 (2007).
Wenzel, A., Grimm, C., Samardzija, M. & Remé, C. E. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Progress. Retin. Eye Res. 24, 275–306 (2005).
Sheedlo, H. J. et al. RPE-derived factors modulate photoreceptor differentiation: a possible role in the retinal stem cell niche. Vitr. Cell Dev. Biol. Anim. 43, 361–370 (2007).
Westenskow, P. D., Kurihara, T. & Friedlander, M. Utilizing Stem Cell-derived RPE Cells as a Therapeutic Intervention for Age-related Macular Degeneration. (Springer, New York, 2014).
Mariotti, C. et al. Comparative study between amniotic-fluid mesenchymal stem cells and retinal pigmented epithelium (RPE) stem cells ability to differentiate towards RPE cells. Cell Tissue Res. 362, 21–31 (2015).
Alfaro, M. P. & Young, P. P. Lessons from genetically altered mesenchymal stem cells (MSCs): candidates for improved MSC-directed myocardial repair. Cell Transplant. 21, 1065 (2012).
Formigli, L. et al. MSCs seeded on bioengineered scaffolds improve skin wound healing in rats. Wound Repair Regen. 23, 115 (2015).
Casiraghi, F., Noris, M. & Remuzzi, G. Immunomodulatory effects of mesenchymal stromal cells in solid organ transplantation. Curr. Opin. Organ Transplant. 15, 731–737 (2010).
Tyndall, A. & Bocelli, C. MSCs for Autoimmune Diseases. In Mesenchymal Stromal Cells. Stem Cell Biology and Regenerative Medicine (eds. Hematti, P. & Keating, A.) (Humana Press, New York, 2013).
Nietomiguel, T. et al. In vitro simulation of corneal epithelium microenvironment induces a corneal epithelial-like cell phenotype from human adipose tissue mesenchymal stem cells. Curr. Eye Res. 38, 933–944 (2013).
Liu, Y. et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2 + ) channel sulfhydration. Cell Stem Cell 15, 66–78 (2014).
Zhao, Y., Wei, H., Kong, G., Shim, W. & Zhang, G. Hydrogen sulfide augments the proliferation and survival of human induced pluripotent stem cell-derived mesenchymal stromal cells through inhibition of BKCa. Cytotherapy 15, 1395 (2013).
Guo, Z., Li, C. S., Wang, C. M., Xie, Y. J. & Wang, A. L. CSE/H2S system protects mesenchymal stem cells from hypoxia and serum deprivation‑induced apoptosis via mitochondrial injury, endoplasmic reticulum stress and PI3K/Akt activation pathways. Mol. Med. Rep. 12, 2128 (2015).
Li, C. et al. Inhibition of the endogenous CSE/H2S system contributes to hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Mol. Med. Rep. 9, 2467–2472 (2014).
Wang, Z. et al. L-Cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H2S pathway. Neuroscience 237, 106–117 (2013).
Zhang, Q. et al. Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget 7, 58089–58104 (2016).
Fukami, K. & Kawabata, A. Hydrogen sulfide and neuronal differentiation: focus on Ca 2 + channels. Nitric Oxide 46, 50–54 (2015).
Liu, F. et al. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes 63, 1763 (2014).
Yang, R., Yu, T., Liu, D., Shi, S. & Zhou, Y. Hydrogen sulfide promotes immunomodulation of gingiva-derived mesenchymal stem cells via the Fas/FasL coupling pathway. Stem Cell Res. Ther. 9, 62 (2018).
Lobb, I. et al. Hydrogen sulfide treatment mitigates renal allograft ischemia reperfusion injury during cold storage and improves early transplant kidney function and survival following allogeneic renal transplantation. J. Urol. 194, 1806–1815 (2015).
Dong et al. H2S preconditioning of human adipose tissue-derived stem cells increases their efficacy in an in vitro model of cell therapy for simulated ischemia. Life Sci. 113, 14–21 (2014).
Acknowledgements
I thank Dr. Ying Wang and Dr. Yufang Shi, for their comments, suggestions and criticism. This work was supported by grants from the National Natural Science Foundation of China (grant nos. 81820108002).
Author information
Authors and Affiliations
Contributions
Y.H. researched documents and wrote the manuscript. Q.S. revised the manuscript. Y.J. and J.Y. designed the study and reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by G. Melino
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, Y., Shang, Q., Yao, J. et al. Hydrogen sulfide: a gaseous signaling molecule modulates tissue homeostasis: implications in ophthalmic diseases. Cell Death Dis 10, 293 (2019). https://doi.org/10.1038/s41419-019-1525-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-019-1525-1