Abstract
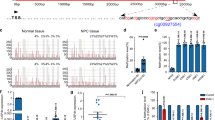
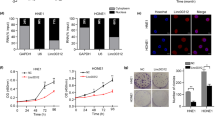
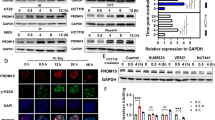
Protein phosphatase 1 catalytic subunit gamma (PPP1CC) promotes DNA repair and tumor development and progression, however, its underlying mechanisms remain unclear. This study investigated the molecular mechanism of PPP1CC’s involvement in DNA repair and the potential clinical implications. High expression of PPP1CC was significantly correlated with radioresistance and poor prognosis in human nasopharyngeal carcinoma (NPC) patients. The mechanistic study revealed that PPP1CC bound to Ku70/Ku80 heterodimers and activated DNA-PKcs by promoting DNA-PK holoenzyme formation, which enhanced nonhomologous end junction (NHEJ) -mediated DNA repair and led to radioresistance. Importantly, BRCA1-BRCA2-containing complex subunit 3 (BRCC3) interacted with PPP1CC to enhance its stability by removing the K48-linked polyubiquitin chain at Lys234 to prevent PPP1CC degradation. Therefore, BRCC3 helped the overexpressed PPP1CC to maintain its high protein level, thereby sustaining the elevation of DNA repair capacity and radioresistance. Our study identified the molecular mechanism by which PPP1CC promotes NHEJ-mediated DNA repair and radioresistance, suggesting that the BRCC3-PPP1CC-Ku70 axis is a potential therapeutic target to improve the efficacy of radiotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data used in this study for protein expression are available in the Clinical Proteome Tumor Analysis Consortium (CPTAC; https://hupo.org/Clinical-Proteome-Tumor-Analysis-Consortium-(CPTAC)). The microarray data used in this study are available in the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession codes GSE12452 and GSE61218. The data used in this study for gene expression profiling interactive analysis (GEPIA; http://gepia.cancer-pku.cn/index.html) are available in The Cancer Genome Atlas (TCGA; https://tcga-data.nci.nih.gov/). The datasets supporting the conclusions of this article are included within the article and its supplementary information files. All original western blots are provided as supplementary material. Data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kockler ZW, Osia B, Lee R, Musmaker K, Malkova A. Repair of DNA breaks by break-induced replication. Annu Rev Biochem. 2021;90:165–91.
Groelly FJ, Fawkes M, Dagg RA, Blackford AN, Tarsounas M. Targeting DNA damage response pathways in cancer. Nat Rev Cancer. 2023;23:78–94.
Awwad SW, Serrano-Benitez A, Thomas JC, Gupta V, Jackson SP. Revolutionizing DNA repair research and cancer therapy with CRISPR-Cas screens. Nat Rev Mol Cell Biol. 2023;24:477–94.
Pilie PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019;16:81–104.
Xu Y, et al. The Largest Chinese Cohort Study indicates homologous recombination pathway gene mutations as another major genetic risk factor for colorectal cancer with heterogeneous clinical phenotypes. Research. 2023;6:0249.
Murmann-Konda T, Soni A, Stuschke M, Iliakis G. Analysis of chromatid-break-repair detects a homologous recombination to non-homologous end-joining switch with increasing load of DNA double-strand breaks. Mutat Res Gen Toxicol Environ Mutagen. 2021;867:503372.
Frank KM, et al. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–7.
Gao Y, et al. A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell. 1998;95:891–902.
Woodbine L, Gennery AR, Jeggo PA. Reprint of “The clinical impact of deficiency in DNA non-homologous end-joining. DNA Repair. 2014;17:9–20.
Stephens PJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40.
Ghezraoui H, et al. Chromosomal translocations in human cells are generated by canonical nonhomologous end-joining. Mol Cell. 2014;55:829–42.
Peycheva M, et al. DNA replication timing directly regulates the frequency of oncogenic chromosomal translocations. Science. 2022;377:1277.
Sishc BJ, Davis AJ. The role of the core non-homologous end joining factors in carcinogenesis and cancer. Cancers. 2017;9:1–30.
Stavridi AK, Appleby R, Liang SK, Blundell TL, Chaplin AK. Druggable binding sites in the multicomponent assemblies that characterise DNA double-strand-break repair through non-homologous end joining. Essays Biochem. 2020;64:791–806.
Chen YK, et al. LRRC31 inhibits DNA repair and sensitizes breast cancer brain metastasis to radiation therapy. Nature Cell Biology. 2020;22:1276.
Chen Y, et al. USP44 regulates irradiation-induced DNA double-strand break repair and suppresses tumorigenesis in nasopharyngeal carcinoma. Nature Communications. 2022;13:501.
Liao JB, et al. Methyltransferase 1 is required for nonhomologous end-joining repair and renders hepatocellular carcinoma resistant to radiotherapy. Hepatology. 2023;77:1896–1910.
Wang XC, et al. Genome-wide RNAi screening identifies RFC4 as a factor that mediates radioresistance in colorectal cancer by facilitating nonhomologous end joining repair. Clin Cancer Res. 2019;25:4567–79.
Zhou W, et al. GTP signaling links metabolism, DNA repair, and responses to genotoxic stress. bioRxiv. 2024;14:158–76.
Réthi-Nagy Z, Abrahám E, Sinka R, Juhász S, Lipinszki Z. Protein phosphatase 4 is required for centrobin function in DNA damage repair. Cells-Basel. 2023;12:2219.
Lee DH, et al. A PP4 phosphatase complex dephosphorylates RPA2 to facilitate DNA repair via homologous recombination. Nat Struct Mol Biol. 2010;17:365–U138.
Ambjorn SM, et al. A complex of BRCA2 and PP2A-B56 is required for DNA repair by homologous recombination. Nat Commun. 2021;12:5748.
Xie WJ, et al. Comprehensive analysis of PPPCs family reveals the clinical significance of PPP1CA and PPP4C in breast cancer. Bioengineered. 2022;13:190–205.
Liu JP, et al. Protein phosphatase PP4 is involved in NHEJ-mediated repair of DNA double-strand breaks. Cell Cycle. 2012;11:2643–9.
Douglas P, et al. Protein phosphatase 6 interacts with the DNA-dependent protein kinase catalytic subunit and dephosphorylates gamma-H2AX. Mol Cell Biol. 2010;30:1368–81.
Yu YM, Pace SM, Allen SR, Deng CX, Hsu LC. A PP1-binding motif present in BRCA1 plays a role in its DNA repair function. Int J Biol Sci. 2008;4:352–61.
Hsu LC. Identification and functional characterization of a PP1-binding site in BRCA1. Biochem Biophys Res Commun. 2007;360:507–12.
Liu Y, Virshup DM, White RL, Hsu LC. Regulation of BRCA1 phosphorylation by interaction with protein phosphatase 1α. Cancer Res. 2002;62:6357–61.
Scully R, et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–75.
Scully R, et al. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435.
Zhong Q, et al. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science. 1999;285:747–50.
Deng CX, Wang RH. Roles of BRCA1 in DNA damage repair: a link between development and cancer. Hum Mol Genet. 2003;12:R113–23.
Hartman AR, Ford JM. BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat Genet. 2002;32:180–4.
Zhu SL, Fisher LA, Bessho T, Peng AM. Protein phosphatase 1 and phosphatase 1 nuclear targeting subunit-dependent regulation of DNA-dependent protein kinase and non-homologous end joining. Nucleic Acids Res. 2017;45:10583–94.
Tu ZW, et al. BRCC3 acts as a prognostic marker in nasopharyngeal carcinoma patients treated with radiotherapy and mediates radiation resistance in vitro. Radiat Oncol. 2015;10:123.
Lee AWM, Ma BBY, Ng WT, Chan ATC. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356.
Wong KCW, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol. 2021;18:679–95.
Qiao H, et al. Association of intratumoral microbiota with prognosis in patients with nasopharyngeal carcinoma from 2 hospitals in China. JAMA Oncol. 2022;8:1301–9.
Zhang PP, et al. Protein C receptor maintains cancer stem cell properties via activating lipid synthesis in nasopharyngeal carcinoma. Signal Transduct Targeted Ther. 2022;7:46.
Li JY, et al. TRIM21 inhibits irradiation-induced mitochondrial DNA release and impairs antitumour immunity in nasopharyngeal carcinoma tumour models. Nat Commun. 2023;14:865.
Jackson L, et al. Influence of family history on penetrance of hereditary cancers in a population setting. Eclinicalmedicine. 2023;64:102159.
Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature. 2008;453:682–6.
Patil A, Nakai K, Nakamura H. HitPredict: a database of quality assessed protein-protein interactions in nine species. Nucleic Acids Res. 2011;39:D744–9.
Yu YL, Yu J, Ge SF, Su Y, Fan XQ. Novel insight into metabolic reprogrammming in cancer radioresistance: a promising therapeutic target in radiotherapy. Int J Biol Sci. 2023;19:811–28.
Jiang K, et al. STC2 activates PRMT5 to induce radioresistance through DNA damage repair and ferroptosis pathways in esophageal squamous cell carcinoma. Redox Biol. 2023;60:102626.
Price JM, Prabhakaran A, West CML. Predicting tumour radiosensitivity to deliver precision radiotherapy. Nat Rev Clin Oncol. 2023;20:83–98.
Wu Y, Song Y, Wang R, Wang T. Molecular mechanisms of tumor resistance to radiotherapy. Mol Cancer. 2023;22:96.
Chen QP, et al. SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 2023;30:137–51.
Jie XH, et al. USP9X-mediated KDM4C deubiquitination promotes lung cancer radioresistance by epigenetically inducing TGF-beta 2 transcription. Cell Death Differ. 2021;28:2095–111.
Zetrini AE, et al. Remodeling tumor immune microenvironment by using polymer-lipid-manganese dioxide nanoparticles with radiation therapy to boost immune response of castration-resistant prostate cancer. Research. 2023;6:0247.
Nambiar DK, Mishra D, Singh RP. Targeting DNA repair for cancer treatment: lessons from PARP inhibitor trials. Oncol Res. 2023;31:405–21.
Wan CH, Wu MM, Zhang SS, Chen YY, Lu CH. alpha 7nAChR-mediated recruitment of PP1 gamma promotes TRAF6/NF-kappa B cascade to facilitate the progression of hepatocellular carcinoma. Mol Carcinogen. 2018;57:1626–39.
Li CS, et al. Overexpression of protein phosphatase 1 gamma (PP1 gamma) is associated with enhanced cell proliferation and poor prognosis in hepatocellular carcinoma. Digest Dis Sci. 2017;62:133–42.
Sogawa K, et al. Enhanced expression of PP1 gamma 1, a catalytic subunit isoform of protein phosphatase type 1, in invasive ductal carcinoma of the breast. Cancer Lett. 1997;112:263–8.
Sogawa K, et al. Enhanced expression of catalytic subunit isoform PP1 gamma 1 of protein phosphatase type 1 associated with malignancy of osteogenic tumor. Cancer Lett. 1995;89:1–6.
Kou Y, Zhang S, Chen X, Hu S. Gene expression profile analysis of colorectal cancer to investigate potential mechanisms using bioinformatics. Onco Targets Ther. 2015;8:745–52.
Malone D, Lardelli RM, Li MQ, David M. Dephosphorylation activates the interferon-stimulated Schlafen family member 11 in the DNA damage response. J Biol Chem. 2019;294:14674–85.
Dobbs TA, Tainer JA, Lees-Miller SP. A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation. DNA Repair. 2010;9:1307–14.
Hammel M, et al. Ku and DNA-dependent protein kinase dynamic conformations and assembly regulate DNA binding and the initial non-homologous end joining complex. J Biol Chem. 2010;285:1414–23.
Dong YS, et al. Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair. Mol Cell. 2003;12:1087–1099.
Chen XW, Arciero CA, Wang CR, Broccoli D, Godwin AK. BRCC36 is essential for ionizing radiation-induced BRCA1 phosphorylation and nuclear foci formation. Cancer Res. 2006;66:5039–46.
Tao H, et al. BRCC3 promotes tumorigenesis of bladder cancer by activating the NF-kappaB signaling pathway through targeting TRAF2. Front Cell Dev Biol. 2021;9:720349.
Hu Y, Zhang Y, Ding M, Xu R. Long noncoding RNA TMPO-AS1/miR-126-5p/BRCC3 axis accelerates gastric cancer progression and angiogenesis via activating PI3K/Akt/mTOR pathway. J Gastroenterol Hepatol. 2021;36:1877–88.
Zhao Y, et al. Hypermethylation of UCHL1 promotes metastasis of nasopharyngeal carcinoma by suppressing degradation of cortactin (CTTN). Cells. 2020;9:559.
Zhao Y, et al. USP2a supports metastasis by tuning TGF-beta signaling. Cell Rep. 2018;22:2442–54.
Liu N, et al. Reduced expression of Dicer11 is associated with poor prognosis in patients with nasopharyngeal carcinoma. Med Oncol. 2013;30:360.
Acknowledgements
The authors thank Prof. M. Zeng, Prof. and Prof. H. Mai (Sun Yat-sen University Cancer Center, China) for human NPC cells, plasmids and technical advice. Professor Liu Ranyi (Sun Yat-sen University Cancer Center, China) kindly contributed reporter constructs for HR, NHEJ, and the I-SceI expression plasmid pCMV-NLS-I-SceI. This work was sponsored by the National Natural Science Foundation of China (81872464), Science and Technology Program of Guangdong Province, China (2021A0505110010), and National High-tech R&D Program of China (863 Program) (2006AA02Z4B4).
Funding
This work was sponsored by the National Natural Science Foundation of China (81872464), Science and Technology Program of Guangdong Province, China (2021A0505110010 and 010192200059), and National High-tech R&D Program of China (863 Program) (2006AA02Z4B4).
Author information
Authors and Affiliations
Contributions
PF, YFX, and YW designed, carried out and analyzed most of the experiments. NL checked the Figures, controlled the experiment steps, and was involved in discussions of the data. YMC provided helped with pathological analysis. YJH, SHZ, and YL helped organize and analyze clinical information and follow-up data. XX, ZLH, and TCJ contributed to several experiments. WD touched up the manuscript. PF wrote the manuscript with input from all other authors. PH and YFX conceived the study, provided scientific directions, and established collaborations. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Sun Yat-sen University Cancer Center’s Institutional Ethical Review Board (GZR2018-100), and the informed consent was obtained from all patients. Animal experiments were approved by the Animal Research Ethics Committee of Sun Yat-sen University Cancer Center and complied with the relevant ethical guidelines (L102012020120M). All research protocols adhered to the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, P., Wang, Y., Liu, N. et al. High expression of PPP1CC promotes NHEJ-mediated DNA repair leading to radioresistance and poor prognosis in nasopharyngeal carcinoma. Cell Death Differ (2024). https://doi.org/10.1038/s41418-024-01287-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41418-024-01287-5