Abstract
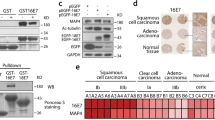
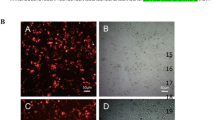
Cervical cancer is the most common gynecologic cancer, etiologically related to persistent infection of human papillomavirus (HPV). Both the host innate immunity system and the invading HPV have developed sophisticated and effective mechanisms to counteract each other. As a central innate immune sensing signaling adaptor, stimulator of interferon genes (STING) plays a pivotal role in antiviral and antitumor immunity, while viral oncoproteins E7, especially from HPV16/18, are responsible for cell proliferation in cervical cancer, and can inhibit the activity of STING as reported. In this report, we find that activation of STING-TBK1 (TANK-binding kinase 1) promotes the ubiquitin-proteasome degradation of E7 oncoproteins to suppress cervical cancer growth. Mechanistically, TBK1 is able to phosphorylate HPV16/18 E7 oncoproteins at Ser71/Ser78, promoting the ubiquitination and degradation of E7 oncoproteins by E3 ligase HUWE1. Functionally, activated STING inhibits cervical cancer cell proliferation via down-regulating E7 oncoproteins in a TBK1-dependent manner and potentially synergizes with radiation to achieve better effects for antitumor. Furthermore, either genetically or pharmacologically activation of STING-TBK1 suppresses cervical cancer growth in mice, which is independent on its innate immune defense. In conclusion, our findings represent a new layer of the host innate immune defense against oncovirus and provide that activating STING/TBK1 could be a promising strategy to treat patients with HPV-positive cervical cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data generated in this study are available within the article and its supplementary data files. The online version contains supplementary material. Any additional information related to this work is available from the corresponding author on reasonable request. The authenticity of this manuscript was validated by uploading the key raw data to the Research Data Deposit public platform (www.researchdata.org.cn).
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50.
Vonsky M, Shabaeva M, Runov A, Lebedeva N, Chowdhury S, Palefsky JM, et al. Carcinogenesis associated with human papillomavirus infection. mechanisms and potential for immunotherapy. Biochemistry (Mosc). 2019;84:782–99.
Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–60.
Gupta S, Kumar P, Das BC. HPV: molecular pathways and targets. Curr Probl Cancer. 2018;42:161–74.
Münger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–98.
Lo Cigno I, Calati F, Albertini S, Gariglio M. Subversion of host innate immunity by human papillomavirus oncoproteins. Pathogens. 2020;9:292.
Bhattacharjee R, Das SS, Biswal SS, Nath A, Das D, Basu A, et al. Mechanistic role of HPV-associated early proteins in cervical cancer: Molecular pathways and targeted therapeutic strategies. Crit Rev Oncol Hematol. 2022;174:103675.
Nagarsheth NB, Norberg SM, Sinkoe AL, Adhikary S, Meyer TJ, Lack JB, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med. 2021;27:419–25.
Zhen S, Lu J, Liu YH, Chen W, Li X. Synergistic antitumor effect on cervical cancer by rational combination of PD1 blockade and CRISPR-Cas9-mediated HPV knockout. Cancer Gene Ther. 2020;27:168–78.
Aarthy M, Kumar D, Giri R, Singh SK. E7 oncoprotein of human papillomavirus: Structural dynamics and inhibitor screening study. Gene. 2018;658:159–77.
Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GWG, Westmoreland D, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–7.
Hu Z, Ding W, Zhu D, Yu L, Jiang X, Wang X, et al. TALEN-mediated targeting of HPV oncogenes ameliorates HPV-related cervical malignancy. J Clin Invest. 2015;125:425–36.
Ritchie C, Carozza JA, Li L. Biochemistry, cell biology, and pathophysiology of the innate immune cGAS-cGAMP-STING pathway. Annu Rev Biochem. 2022;91:599–628.
Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8.
Ma Z, Damania B. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe. 2016;19:150–8.
Cheng Z, Dai T, He X, Zhang Z, Xie F, Wang S, et al. The interactions between cGAS-STING pathway and pathogens. Signal Transduct Target Ther. 2020;5:91.
Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–92.
Garland KM, Sheehy TL, Wilson JT. Chemical and biomolecular strategies for STING pathway activation in cancer immunotherapy. Chemical Reviews. 2022;122:5977–6039.
Yum S, Li M, Chen ZJ. Old dogs, new trick: classic cancer therapies activate cGAS. Cell Res. 2020;30:639–48.
Poltorak A, Kurmyshkina O, Volkova T. Stimulator of interferon genes (STING): A “new chapter” in virus-associated cancer research. Lessons from wild-derived mouse models of innate immunity. Cytokine Growth Factor Rev. 2016;29:83–91.
Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang S, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564:439–43.
Pan B-S, Perera SA, Piesvaux JA, Presland JP, Schroeder GK, Cumming JN, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020;369:eaba6098.
Chin EN, Yu C, Vartabedian VF, Jia Y, Kumar M, Gamo AM, et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science. 2020;369:993–9.
McIntosh JA, Liu Z, Andresen BM, Marzijarani NS, Moore JC, Marshall NM, et al. A kinase-cGAS cascade to synthesize a therapeutic STING activator. Nature. 2022;603:439–44.
Lau L, Gray EE, Brunette RL, Stetson DB. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. 2015;350:568–71.
Lou M, Huang D, Zhou Z, Shi X, Wu M, Rui Y, et al. DNA virus oncoprotein HPV18 E7 selectively antagonizes cGAS‐STING‐triggered innate immune activation. J Med Virol. 2022;95:e28310.
Luo X, Donnelly CR, Gong W, Heath BR, Hao Y, Donnelly LA, et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J Clin Invest. 2020;130:1635–52.
Ranoa DRE, Widau RC, Mallon S, Parekh AD, Nicolae CM, Huang X, et al. STING promotes homeostasis via regulation of cell proliferation and chromosomal stability. Cancer Res. 2019;79:1465–79.
Liu S, Guan W. STING signaling promotes apoptosis, necrosis, and cell death: an overview and update. Mediat Inflamm. 2018;2018:1202797.
Gao Y, Zheng X, Chang B, Lin Y, Huang X, Wang W, et al. Intercellular transfer of activated STING triggered by RAB22A-mediated non-canonical autophagy promotes antitumor immunity. Cell Res. 2022;32:1086–104.
Yi J, Lu G, Li L, Wang X, Cao L, Lin M, et al. DNA damage-induced activation of CUL4B targets HUWE1 for proteasomal degradation. Nucleic Acids Res. 2015;43:4579–90.
Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA, et al. Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548:466–70.
Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–52.
Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Sanchez GAM, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–18.
Melki I, Rose Y, Uggenti C, Van Eyck L, Fremond ML, Kitabayashi N, et al. Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J Allergy Clin Immunol. 2017;140:543–52. e5
Zhang C, Shang G, Gui X, Zhang X, Bai XC, Chen ZJ. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567:394–8.
Massimi P, Banks L. Differential phosphorylation of the HPV-16 E7 oncoprotein during the cell cycle. Virology. 2000;276:388–94.
Tu D, Zhu Z, Zhou AY, Yun CH, Lee KE, Toms AV, et al. Structure and ubiquitination-dependent activation of TANK-binding kinase 1. Cell Rep. 2013;3:747–58.
Shi F, Su J, Wang J, Liu Z, Wang T. Activation of STING inhibits cervical cancer tumor growth through enhancing the anti-tumor immune response. Mol Cell Biochem. 2021;476:1015–24.
Yang A, Jeang J, Cheng K, Cheng T, Yang B, Wu TC, et al. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines. 2016;15:989–1007.
Gogoi H, Mansouri S, Jin L. The age of cyclic dinucleotide vaccine adjuvants. Vaccines (Basel). 2020;8:453.
Liu Z, Zhou J, Xu W, Deng W, Wang Y, Wang M, et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs. Cell Res. 2022;32:269–87.
Acknowledgements
We thank Dr. Ying Sun (Sun Yat-sen University Cancer Center) for helpful suggestions. We thank members of Dr. Kang’s laboratory for providing helpful suggestions on this study. This work was supported by the National Key R&D Program of China (2021YFA1300601), and the grants from the National Natural Science Foundation of China (82002428, 81972430, 82103155, 32100544, 82341015, 82030090)
Author information
Authors and Affiliations
Contributions
XH, TK and YG conceived the project, designed the experiments and wrote the manuscript. XH, YG, LH and BX performed most of the experiments and analyzed the data. XH, YO, FC and JL performed the statistical analyses. XH, YG, LH and XZ performed the animal experiments. DW, YW and RZ assisted with experiments and provided technical help. XC provided comments and revised the manuscript. All authors have reviewed the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Animal studies were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals and the Principles for the Utilization and Care of Vertebrate Animals and approved by the Animal Research Committee of Sun Yat-sen University Cancer Center (SYSUCC) (Approval no. L102042020100L).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Huo, L., Xiao, B. et al. Activating STING/TBK1 suppresses tumor growth via degrading HPV16/18 E7 oncoproteins in cervical cancer. Cell Death Differ 31, 78–89 (2024). https://doi.org/10.1038/s41418-023-01242-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01242-w
This article is cited by
-
Interferon-γ-induced GBP1 is an inhibitor of human papillomavirus 18
BMC Women's Health (2024)