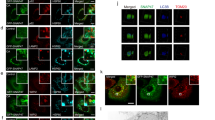
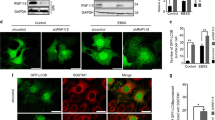
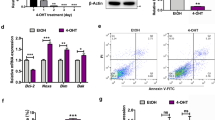
Abstract
Autophagy serves as a pro-survival mechanism for a cell or a whole organism to cope with nutrient stress. Our understanding of the molecular regulation of this fusion event remains incomplete. Here, we identified RUNDC1 as a novel ATG14-interacting protein, which is highly conserved across vertebrates, including zebrafish and humans. By gain and loss of function studies, we demonstrate that RUNDC1 negatively modulates autophagy by blocking fusion between autophagosomes and lysosomes via inhibiting the assembly of the STX17-SNAP29-VAMP8 complex both in human cells and the zebrafish model. Moreover, RUNDC1 clasps the ATG14-STX17-SNAP29 complex via stimulating ATG14 homo-oligomerization to inhibit ATG14 dissociation. This also prevents VAMP8 from binding to STX17-SNAP29. We further identified that phosphorylation of RUNDC1 Ser379 is crucial to inhibit the assembly of the STX17-SNAP29-VAMP8 complex via promoting ATG14 homo-oligomerization. In line with our findings, RunDC1 is crucial for zebrafish in their response to nutrient-deficient conditions. Taken together, our findings demonstrate that RUNDC1 is a negative regulator of autophagy that restricts autophagosome fusion with lysosomes by clasping the ATG14-STX17-SNAP29 complex to hinder VAMP8 binding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data analyzed during this study are included in this published article and the supplemental data files. Additional supporting data are available from the corresponding authors upon reasonable request.
References
Kang C, You YJ, Avery L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 2007;21:2161–71.
Levine B, Kroemer G. Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell Death Differ. 2009;16:1–2.
Yamamoto H, Zhang S, Mizushima N. Autophagy genes in biology and disease. Nat Rev Genet. 2023;24:382–400.
Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12.
Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023;24:167–85.
Diao J, Liu R, Rong Y, Zhao M, Zhang J, Lai Y, et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520:563–6.
Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11:385–96.
Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–93.
Hurley JH, Young LN. Mechanisms of autophagy initiation. Annu Rev Biochem. 2017;86:225–44.
Liu R, Zhi X, Zhong Q. ATG14 controls SNARE-mediated autophagosome fusion with a lysosome. Autophagy. 2015;11:847–9.
Zhang H, Ge S, Ni B, He K, Zhu P, Wu X, et al. Augmenting ATG14 alleviates atherosclerosis and inhibits inflammation via promotion of autophagosome-lysosome fusion in macrophages. Autophagy. 2021;17:4218–30.
Moreau K, Renna M, Rubinsztein DC. Connections between SNAREs and autophagy. Trends Biochem Sci. 2013;38:57–63.
Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol. 2021;22:733–50.
Tian X, Teng J, Chen J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy. 2021;17:2680–88.
Zhao YG, Zhang H. Autophagosome maturation: An epic journey from the ER to lysosomes. J Cell Biol. 2019;218:757–70.
Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–69.
Llanos S, Efeyan A, Monsech J, Dominguez O, Serrano M. A high-throughput loss-of-function screening identifies novel p53 regulators. Cell Cycle. 2006;5:1880–5.
Jalali A, Amirian ES, Bainbridge MN, Armstrong GN, Liu Y, Tsavachidis S, et al. Targeted sequencing in chromosome 17q linkage region identifies familial glioma candidates in the Gliogene Consortium. Sci Rep. 2015;5:8278.
McEwan DG, Dikic I. The Three Musketeers of Autophagy: phosphorylation, ubiquitylation and acetylation. Trends Cell Biol. 2011;21:195–201.
Xie Y, Kang R, Sun X, Zhong M, Huang J, Klionsky DJ, et al. Posttranslational modification of autophagy-related proteins in macroautophagy. Autophagy. 2015;11:28–45.
Nguyen A, Lugarini F, David C, Hosnani P, Alagoz C, Friedrich A, et al. Metamorphic proteins at the basis of human autophagy initiation and lipid transfer. Mol Cell. 2023;83:2077–90.e12.
He C, Bartholomew CR, Zhou W, Klionsky DJ. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy. 2009;5:520–6.
Sahani MH, Itakura E, Mizushima N. Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy. 2014;10:431–41.
Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26.
Suster ML, Kikuta H, Fau - Urasaki A, Urasaki A, Fau - Asakawa K, Asakawa K, et al. Transgenesis in zebrafish with the tol2 transposon system. Methods Mol Biol. 2009;561:41–63.
Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–4.
Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73.
Nakamura S, Yoshimori T. New insights into autophagosome-lysosome fusion. J Cell Sci. 2017;130:1209–16.
Diao J, Li L, Lai Y, Zhong Q. In Vitro Reconstitution of Autophagosome-Lysosome Fusion. Methods Enzymol. 2017;587:365–76.
Shen Q, Shi Y, Liu J, Su H, Huang J, Zhang Y, et al. Acetylation of STX17 (syntaxin 17) controls autophagosome maturation. Autophagy. 2021;17:1157–69.
Zhang X, Wang L, Lak B, Li J, Jokitalo E, Wang Y. GRASP55 senses glucose deprivation through O-GlcNAcylation to promote autophagosome-lysosome fusion. Dev Cell. 2018;45:245–61.e6.
Guo B, Liang Q, Li L, Hu Z, Wu F, Zhang P, et al. O-GlcNAc-modification of SNAP-29 regulates autophagosome maturation. Nat Cell Biol. 2014;16:1215–26.
Shen Q, Shi Y, Liu J, Su H, Huang J, Zhang Y, et al. Acetylation of STX17 (syntaxin 17) controls autophagosome maturation. Autophagy. 2021;17:1157–69.
Pellegrini FR, De Martino S, Fianco G, Ventura I, Valente D, Fiore M, et al. Blockage of autophagosome-lysosome fusion through SNAP29 O-GlcNAcylation promotes apoptosis via ROS production. Autophagy. 2023;19:2078–93.
Pearlman SM, Serber Z, Ferrell JE Jr. A mechanism for the evolution of phosphorylation sites. Cell. 2011;147:934–46.
Ravanan P, Srikumar IF, Talwar P. Autophagy: the spotlight for cellular stress responses. Life Sci. 2017;188:53–67.
Masselink W. Crispants take the spotlight. Lab Anim. 2021;50:95–6.
Moravec CE, Voit GC, Otterlee J, Pelegri F. Identification of maternal-effect genes in zebrafish using maternal crispants. Development. 2021;148:dev199536.
Tian X, Teng J, Chen J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy. 2021;17:2680–8.
Gibson TJ, Seiler M, Veitia RA. The transience of transient overexpression. Nat Methods. 2013;10:715–21.
Obara K, Ohsumi Y. Atg14: a key player in orchestrating autophagy. Int J Cell Biol. 2011;2011:713435.
Zhao Y, Zou Z, Sun D, Li Y, Sinha SC, Yu L, et al. GLIPR2 is a negative regulator of autophagy and the BECN1-ATG14-containing phosphatidylinositol 3-kinase complex. Autophagy. 2021;17:2891–904.
Itakura E, Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6:764–76.
Fan W, Nassiri A, Zhong Q. Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L). Proc Natl Acad Sci USA. 2011;108:7769–74.
Bernard A, Klionsky DJ. Toward an understanding of autophagosome-lysosome fusion: the unsuspected role of ATG14. Autophagy. 2015;11:583–4.
Yoon TY, Munson M. SNARE complex assembly and disassembly. Curr Biol. 2018;28:R367–R420.
Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93.
Zhou X-Y, Zhang T, Ren L, Wu J-J, Wang W, Liu J-X. Copper elevated embryonic hemoglobin through reactive oxygen species during zebrafish erythrogenesis. Aquat Toxicol. 2016;175:1–11.
Subramaniam A, Žemaitis K, Talkhoncheh MS, Yudovich D, Bäckström A, Debnath S, et al. Lysine-specific demethylase 1A restricts ex vivo propagation of human HSCs and is a target of UM171. Blood. 2020;136:2151–61.
Acknowledgements
We are grateful to Prof. Daniel Klionsky and Prof. Annemarie Meijer for sharing the GFP-Lc3 transgenic zebrafish line. We also thank the Hubei University of Technology for financial and equipment support for this research.
Funding
This work was supported by the National Natural Science Foundation of China (32000523 to RZ, 82273970 and 32070726 to JFT, 31871176 to XZC, 32270768 to CFZ), International Science and Technology Cooperation Project of Hubei Province (2022EHB038 to CFZ), Wuhan Science and Technology Project (2022020801020272 to C.F.Z) and Innovation Group Project of Hubei Province (2023AFA026).
Author information
Authors and Affiliations
Contributions
RZ, YY, CH, XZ and SW performed molecular biology and zebrafish experiments and write the main manuscript. JT and CZ designed the whole project and supervised all experiments. RZ, YY, CH, XZ conducted all experiments and analyzed the data. VC, NL, YR, LC, LY, GZ, LS, DY, XF, HY, LH, SX, GD, DA, MM, XC, and CM contributed with critical feedback on the presented work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experimental protocol for animal studies was reviewed and approved by the institutional animal care and use committee of the Hubei University of Technology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, R., Yang, Y., He, C. et al. RUNDC1 inhibits autolysosome formation and survival of zebrafish via clasping ATG14-STX17-SNAP29 complex. Cell Death Differ 30, 2231–2248 (2023). https://doi.org/10.1038/s41418-023-01215-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01215-z