Abstract
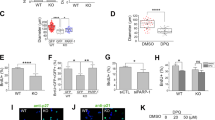
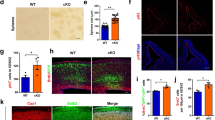
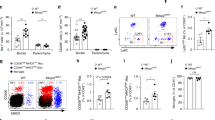
Embryonic neurogenesis is tightly regulated by multiple factors to ensure the precise development of the cortex. Deficiency in neurogenesis may result in behavioral abnormalities. Pd1 is a well-known inhibitory immune molecule, but its function in brain development remains unknown. Here, we find brain specific deletion of Pd1 results in abnormal cortical neurogenesis, including enhanced proliferation of neural progenitors and reduced neuronal differentiation. In addition, neurons in Pd1 knockout mice exhibit abnormal morphology, both the total length and the number of primary dendrites were reduced. Moreover, Pd1cKO mice exhibit depressive-like behaviors, including immobility, despair, and anhedonia. Mechanistically, Pd1 regulates embryonic neurogenesis by targeting Pax3 through the β-catenin signaling pathway. The constitutive expression of Pax3 partly rescues the deficiency of neurogenesis in the Pd1 deleted embryonic brain. Besides, the administration of β-catenin inhibitor, XAV939, not only rescues abnormal brain development but also ameliorates depressive-like behaviors in Pd1cKO mice. Simultaneously, Pd1 plays a similar role in human neural progenitor cells (hNPCs) proliferation and differentiation. Taken together, our findings reveal the critical role and regulatory mechanism of Pd1 in embryonic neurogenesis and behavioral modulation, which could contribute to understanding immune molecules in brain development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq data are available in GEO with the following accession number: GSE207712.
References
Boku S, Nakagawa S, Toda H, Hishimoto A. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72:3–12.
Barch DM, Tillman R, Kelly D, Whalen D, Gilbert K, Luby JL. Hippocampal volume and depression among young children. Psychiatry Res Neuroimaging. 2019;288:21–8.
Maggio N, Vlachos A. Tumor necrosis factor (TNF) modulates synaptic plasticity in a concentration-dependent manner through intracellular calcium stores. J Mol Med. 2018;96:1039–47.
Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–5.
Wei H, Zou H, Sheikh AM, Malik M, Dobkin C, Brown WT, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78.
Glynn MW, Elmer BM, Garay PA, Liu XB, Needleman LA, El-Sabeawy F, et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci. 2011;14:442–51.
Liang Q, Su L, Zhang D, Jiao J. CD93 negatively regulates astrogenesis in response to MMRN2 through the transcriptional repressor ZFP503 in the developing brain. Proc Natl Acad Sci USA. 2020;117:9413–22.
Zhang D, Liu C, Li H, Jiao J. Deficiency of STING signaling in embryonic cerebral cortex leads to neurogenic abnormalities and autistic-like behaviors. Adv Sci. 2020;7:2002117.
Chen G, Kim YH, Li H, Luo H, Liu DL, Zhang ZJ, et al. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat Neurosci. 2017;20:917–26.
Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med. 2016;22:135–7.
Leroy C, Landais E, Briault S, David A, Tassy O, Gruchy N, et al. The 2q37-deletion syndrome: an update of the clinical spectrum including overweight, brachydactyly and behavioural features in 14 new patients. Eur J Hum Genet. 2013;21:602–12.
Zhao T, Gan Q, Stokes A, Lassiter RN, Wang Y, Chan J, et al. beta-catenin regulates Pax3 and Cdx2 for caudal neural tube closure and elongation. Development. 2014;141:148–57.
Han Y. Liu D, and Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727–42.
Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109.
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–42.
Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000.
Winter G, Hart RA, Charlesworth RPG, Sharpley CF. Gut microbiome and depression: what we know and what we need to know. Rev Neurosci. 2018;29:629–43.
Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9.
Ji F, Wang W, Feng C, Gao F, Jiao J. Brain-specific Wt1 deletion leads to depressive-like behaviors in mice via the recruitment of Tet2 to modulate Epo expression. Mol Psychiatry. 2021;26:4221–33.
Li H, Zhu Y, Morozov YM, Chen X, Page SC, Rannals MD, et al. Disruption of TCF4 regulatory networks leads to abnormal cortical development and mental disabilities. Mol Psychiatry. 2019;24:1235–46.
Deng ZF, Zheng HL, Chen JG, Luo Y, Xu JF, Zhao G, et al. miR-214-3p targets beta-catenin to regulate depressive-like behaviors induced by chronic social defeat stress in mice. Cereb Cortex. 2019;29:1509–19.
Karege F, Perroud N, Burkhardt S, Fernandez R, Ballmann E, La Harpe R, et al. Protein levels of beta-catenin and activation state of glycogen synthase kinase-3beta in major depression. A study with postmortem prefrontal cortex. J Affect Disord. 2012;136:185–8.
Gould TD, O’Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189:117–25.
Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–9.
Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–43.
Li Y, Jiao J. Histone chaperone HIRA regulates neural progenitor cell proliferation and neurogenesis via beta-catenin. J Cell Biol. 2017;216:1975–92.
Geerlings MI, Sigurdsson S, Eiriksdottir G, Garcia ME, Harris TB, Sigurdsson T, et al. Associations of current and remitted major depressive disorder with brain atrophy: the AGES-Reykjavik Study. Psychol Med. 2013;43:317–28.
Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17.
Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66.
Zhao J, Roberts A, Wang Z, Savage J, Ji RR. Emerging role of PD-1 in the central nervous system and brain diseases. Neurosci Bull. 2021;37:1188–202.
Zhao J, Bang S, Furutani K, McGinnis A, Jiang C, Roberts A, et al. PD-L1/PD-1 checkpoint pathway regulates hippocampal neuronal excitability and learning and memory behavior. Neuron. 2023;S0896-6273:00395-1. https://doi.org/10.1016/j.neuron.2023.05.022.
Acknowledgements
We thank all members of our lab for their valuable suggestions. We also thank Shiwen Li, Lijuan Wang, Hua Qin, and Xili Zhu for their help in confocal imaging and behavioral analysis. This work was supported by grants from the National Key R&D Program of China (2019YFA0110301 and 2021YFA1101402), the National Natural Science Foundation of China (32230040, 81825006, and 92149304), and Guangdong Basic and Applied Basic Research Foundation (2022B1515120026).
Author information
Authors and Affiliations
Contributions
FJ performed the experiments, analyzed the results, and wrote the manuscript. CF constructed the plasmids of Pd1 and Pdl1. JQ, CW, LS, WW, MZ, HL, LM, WL, CL, ZT, and BH provided help in the experiment performance. DZ provided help in human NSCs culture. FJ and JX supervised the project. JJ supervised the project and provided funding support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, F., Feng, C., Qin, J. et al. Brain-specific Pd1 deficiency leads to cortical neurogenesis defects and depressive-like behaviors in mice. Cell Death Differ 30, 2053–2065 (2023). https://doi.org/10.1038/s41418-023-01203-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01203-3