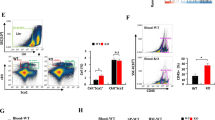
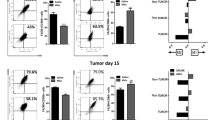
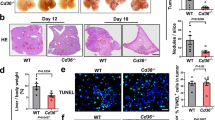
Abstract
Macrophages are usually educated to tumor-associated macrophages (TAMs) in cancer with pro-tumor functions by tumor microenvironment (TME) and TAM reprogramming has been proposed as a potential tumor immunotherapy strategy. We recently demonstrated the critical role of Zinc-fingers and homeoboxes 2 (Zhx2) in macrophages’ metabolic programming. However, whether Zhx2 is responsible for macrophage polarization and TAMs reprogramming is largely unknown. Here, we show that Zhx2 controls macrophage polarization under the inflammatory stimulus and TME. Myeloid-specific deletion of Zhx2 suppresses LPS-induced proinflammatory polarization but promotes IL-4 and TME-induced anti-inflammatory and pro-tumoral phenotypes in murine liver tumor models. Factors in TME, especially lactate, markedly decrease the expression of Zhx2 in TAMs, leading to the switch of TAMs to pro-tumor phenotype and consequent cancer progression. Notably, reduced ZHX2 expression in TAM correlates with poor survival of HCC patients. Mechanistic studies reveal that Zhx2 associates with NF-κB p65 and binds to the Irf1 promoter, leading to transcriptional activation of Irf1 in macrophages. Zhx2 functions in maintaining macrophage polarization by regulating Irf1 transcription, which may be a potential target for macrophage-based cancer immunotherapy.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA sequencing raw data have been deposited in NCBI Sequence Read Archive (SRA) database with the accession number: PRJNA598552. Uncropped original western blots and relevant data are provided in the Supplementary files. The data generated in this study are available upon reasonable request from the corresponding author.
References
Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radio. 2018;43:13–25.
Jindal A, Thadi A, Shailubhai K. Hepatocellular carcinoma: etiology and current and future drugs. J Clin Exp Hepatol. 2019;9:221–32.
Chan S, Wong V, Qin S, Chan H. Infection and cancer: the case of hepatitis B. J Clin Oncol. 2016;34:83–90.
Yang Y, Kim S, Seki E. Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Semin Liver Dis. 2019;39:26–42.
Ding W, Tan Y, Qian Y, Xue W, Wang Y, Jiang P, et al. Clinicopathologic and prognostic significance of tumor-associated macrophages in patients with hepatocellular carcinoma: a meta-analysis. PloS One. 2019;14:e0223971.
Li Z, Wu T, Zheng B, Chen L. Individualized precision treatment: targeting TAM in HCC. Cancer Lett. 2019;458:86–91.
Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–12.
Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–44.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
Malyshev I, Malyshev Y. Current concept and update of the macrophage plasticity concept: intracellular mechanisms of reprogramming and M3 macrophage “switch” phenotype. BioMed Res. Int. 2015;2015:341308.
Gordon S, Martinez F. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604.
Vitale I, Manic G, Coussens L, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30:36–50.
DeNardo D, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–82.
Heusinkveld M, van der Burg S. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216.
Baer C, Squadrito M, Laoui D, Thompson D, Hansen S, Kiialainen A, et al. Suppression of microRNA activity amplifies IFN-γ-induced macrophage activation and promotes anti-tumour immunity. Nat cell Biol. 2016;18:790–802.
Cassetta L, Pollard J. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904.
Kawata H, Yamada K, Shou Z, Mizutani T, Miyamoto K. The mouse zinc-fingers and homeoboxes (ZHX) family; ZHX2 forms a heterodimer with ZHX3. Gene. 2003;323:133–40.
Nagel S, Ehrentraut S, Meyer C, Kaufmann M, Drexler HG, MacLeod RA. Aberrantly expressed OTX homeobox genes deregulate B-cell differentiation in hodgkin lymphoma. PLoS One. 2015;10:e0138416.
Zhang J, Wu T, Simon J, Takada M, Saito R, Fan C, et al. VHL substrate transcription factor ZHX2 as an oncogenic driver in clear cell renal cell carcinoma. Science. 2018;361:290–5.
Yue X, Zhang Z, Liang X, Gao L, Zhang X, Zhao D, et al. Zinc fingers and homeoboxes 2 inhibits hepatocellular carcinoma cell proliferation and represses expression of Cyclins A and E. Gastroenterology. 2012;142:1559–70.e1552.
Lin Q, Wu Z, Yue X, Yu X, Wang Z, Song X, et al. ZHX2 restricts hepatocellular carcinoma by suppressing stem cell-like traits through KDM2A-mediated H3K36 demethylation. EBioMedicine. 2020;53:102676.
Wu Z, Ma H, Wang L, Song X, Zhang J, Liu W, et al. Tumor suppressor ZHX2 inhibits NAFLD-HCC progression via blocking LPL-mediated lipid uptake. Cell Death Differ. 2020;27:1693–708.
Tan S, Guo X, Li M, Wang T, Wang Z, Li C, et al. Transcription factor Zhx2 restricts NK cell maturation and suppresses their antitumor immunity. J Exp Med. 2021;218:e20210009.
Espinal-Enríquez J, González-Terán D, Hernández-Lemus E. The transcriptional network structure of a myeloid cell: a computational approach. Int J Genom. 2017;2017:4858173.
Erbilgin A, Seldin MM, Wu X, Mehrabian M, Zhou Z, Qi H, et al. Transcription factor Zhx2 deficiency reduces atherosclerosis and promotes macrophage apoptosis in mice. Arterioscler Thromb Vasc Biol. 2018;38:2016–27.
Wang Z, Kong L, Tan S, Zhang Y, Song X, Wang T, et al. Zhx2 accelerates sepsis by promoting macrophage glycolysis via Pfkfb3. J Immunol. 2020;204:2232–41.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55.
Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799–820.
Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39.
Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis V. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022;23:1148–56.
Li Z, Li H, Zhao Z, Zhu W, Feng P, Zhu X, et al. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARδ signalling-mediated alternative activation of macrophages. J Exp Clin Cancer Res. 2019;38:469.
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63.
Fujii M, Shibazaki Y, Wakamatsu K, Honda Y, Kawauchi Y, Suzuki K, et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol. 2013;46:141–52.
Yamamoto M, Xin B, Watanabe K, Ooshio T, Fujii K, Chen X, et al. Oncogenic determination of a broad spectrum of phenotypes of hepatocyte-derived mouse liver tumors. Am J Pathol. 2017;187:2711–25.
Kang K, Park S, Chen J, Qiao Y, Giannopoulou E, Berg K, et al. Interferon-γ represses M2 gene expression in human macrophages by disassembling enhancers bound by the transcription factor MAF. Immunity. 2017;47:235–50.e234.
Mootha V, Lindgren C, Eriksson K, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73.
Cheng Q, Ohta S, Sheu K, Spreafico R, Adelaja A, Taylor B, et al. NF-κB dynamics determine the stimulus specificity of epigenomic reprogramming in macrophages. Science. 2021;372:1349–53.
Liao Y, Hua Y, Li Y, Zhang C, Yu W, Guo P, et al. CRSP8 promotes thyroid cancer progression by antagonizing IKKα-induced cell differentiation. Cell Death Differ. 2021;28:1347–63.
Ngambenjawong C, Gustafson H, Pun S. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–21.
Chen D, Ning W, Jiang Z, Peng Z, Zhu L, Zhuang S, et al. Glycolytic activation of peritumoral monocytes fosters immune privilege via the PFKFB3-PD-L1 axis in human hepatocellular carcinoma. J Hepatol. 2019;71:333–43.
Morrissey S, Zhang F, Ding C, Montoya-Durango D, Hu X, Yang C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021; 33:2040–58.e2010.
Wei G, Sun H, Dong K, Hu L, Wang Q, Zhuang Q, et al. The thermogenic activity of adjacent adipocytes fuels the progression of ccRCC and compromises anti-tumor therapeutic efficacy. Cell Metab. 2021;33:2021–39.e2028.
Zhang J, Muri J, Fitzgerald G, Gorski T, Gianni-Barrera R, Masschelein E, et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 2020;31:1136–53.e1137.
van der Vorst E, Theodorou K, Wu Y, Hoeksema M, Goossens P, Bursill C, et al. High-density lipoproteins exert pro-inflammatory effects on macrophages via passive cholesterol depletion and PKC-NF-κB/STAT1-IRF1 signaling. Cell Metab. 2017;25:197–207.
Piccolo V, Curina A, Genua M, Ghisletti S, Simonatto M, Sabò A, et al. Opposing macrophage polarization programs show extensive epigenomic and transcriptional cross-talk. Nat Immunol. 2017;18:530–40.
Huang R, Hu Z, Chen X, Cao Y, Li H, Zhang H, et al. The transcription factor SUB1 is a master regulator of the macrophage TLR response in atherosclerosis. Adv Sci. 2021;8:e2004162.
Eckhardt I, Weigert A, Fulda S. Identification of IRF1 as critical dual regulator of Smac mimetic-induced apoptosis and inflammatory cytokine response. Cell Death Dis. 2014;5:e1562.
Moschonas A, Kouraki M, Knox P, Thymiakou E, Kardassis D, Eliopoulos A. CD40 induces antigen transporter and immunoproteasome gene expression in carcinomas via the coordinated action of NF-kappaB and of NF-kappaB-mediated de novo synthesis of IRF-1. Mol Cell Biol. 2008;28:6208–22.
Andersen P, Pedersen M, Woetmann A, Villingshøj M, Stockhausen M, Odum N, et al. EGFR induces expression of IRF-1 via STAT1 and STAT3 activation leading to growth arrest of human cancer cells. Int J Cancer. 2008;122:342–9.
Wang C, Cigliano A, Jiang L, Li X, Fan B, Pilo M, et al. 4EBP1/eIF4E and p70S6K/RPS6 axes play critical and distinct roles in hepatocarcinogenesis driven by AKT and N-Ras proto-oncogenes in mice. Hepatology. 2015;61:200–13.
Acknowledgements
This study was supported in part by the National Key Research and Development Program (2021YFC2300603), the National Science Foundation of China (Key program 81830017, 8223056, 32200742), Taishan Scholarship (No.tspd20181201), Major Basic Research Project of Shandong Natural Science Foundation (No. ZR2020ZD12), the Shandong Provincial Natural Science Foundation (ZR2021QH322, ZR2019PH023) and Collaborative Innovation Centre of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong. We also thank the Translational Medicine Core Facility of Shandong University for the consultation and instrument availability that supported this work.
Author information
Authors and Affiliations
Contributions
CM, ST, and ZW formulated the study concept and designed the studies. ST, ZW, NL, XG, YZ, HM, XP, and YZ performed the experiments. ST, ZW, and CM analyzed the results. CL, LG, TL, XL, and CM interpreted the results. ST, ZW, and CM wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, S., Wang, Z., Li, N. et al. Transcription factor Zhx2 is a checkpoint that programs macrophage polarization and antitumor response. Cell Death Differ 30, 2104–2119 (2023). https://doi.org/10.1038/s41418-023-01202-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01202-4