Abstract
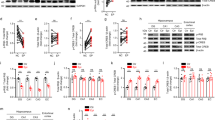
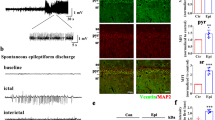
Temporal lobe epilepsy (TLE) is the most common and severe form of epilepsy in adults; however, its underlying pathomechanisms remain elusive. Dysregulation of ubiquitination is increasingly recognized to contribute to the development and maintenance of epilepsy. Herein, we observed for the first time that potassium channel tetramerization domain containing 13 (KCTD13) protein, a substrate-specific adapter for cullin3-based E3 ubiquitin ligase, was markedly down-regulated in the brain tissue of patients with TLE. In a TLE mouse model, the protein expression of KCTD13 dynamically changed during epileptogenesis. Knockdown of KCTD13 in the mouse hippocampus significantly enhanced seizure susceptibility and severity, whereas overexpression of KCTD13 showed the opposite effect. Mechanistically, GluN1, an obligatory subunit of N-methyl-D-aspartic acid receptors (NMDARs), was identified as a potential substrate protein of KCTD13. Further investigation revealed that KCTD13 facilitates lysine-48-linked polyubiquitination of GluN1 and its degradation through the ubiquitin-proteasome pathway. Besides, the lysine residue 860 of GluN1 is the main ubiquitin site. Importantly, dysregulation of KCTD13 affected membrane expression of glutamate receptors and impaired glutamate synaptic transmission. Systemic administration of the NMDAR inhibitor memantine significantly rescued the epileptic phenotype aggravated by KCTD13 knockdown. In conclusion, our results demonstrated an unrecognized pathway of KCTD13-GluN1 in epilepsy, suggesting KCTD13 as a potential neuroprotective therapeutic target for epilepsy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data supporting the conclusions in the paper are present in the paper and the Supplementary Materials. Additional data are available from the corresponding author.
References
Devinsky O, Vezzani A, O’Brien TJ, Jette N, Scheffer IE, de Curtis M, et al. Epilepsy. Nat Rev Dis Primers. 2018;4:18024.
Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701.
Shlobin NA, Sander JW. Learning from the comorbidities of epilepsy. Curr Opin Neurol. 2022;35:175–80.
Vinti V, Dell’Isola GB, Tascini G, Mencaroni E, Cara GD, Striano P, et al. Temporal lobe epilepsy and psychiatric comorbidity. Front Neurol. 2021;12:775781.
Ren E, Curia G. Synaptic reshaping and neuronal outcomes in the temporal lobe epilepsy. Int J Mol Sci. 2021;22:3860.
Pfisterer U, Petukhov V, Demharter S, Meichsner J, Thompson JJ, Batiuk MY, et al. Identification of epilepsy-associated neuronal subtypes and gene expression underlying epileptogenesis. Nat Commun. 2020;11:5038.
Guelfi S, Botia JA, Thom M, Ramasamy A, Perona M, Stanyer L, et al. Transcriptomic and genetic analyses reveal potential causal drivers for intractable partial epilepsy. Brain. 2019;142:1616–30.
Mabb AM. Historical perspective and progress on protein ubiquitination at glutamatergic synapses. Neuropharmacology. 2021;196:108690.
Poliquin S, Kang JQ. Disruption of the ubiquitin-proteasome system and elevated endoplasmic reticulum stress in epilepsy. Biomedicines. 2022;10:647.
Zhu J, Tsai NP. Ubiquitination and E3 ubiquitin ligases in rare neurological diseases with comorbid epilepsy. Neuroscience. 2020;428:90–9.
Li C, Beauregard-Lacroix E, Kondratev C, Rousseau J, Heo AJ, Neas K, et al. UBR7 functions with UBR5 in the Notch signaling pathway and is involved in a neurodevelopmental syndrome with epilepsy, ptosis, and hypothyroidism. Am J Hum Genet. 2021;108:134–47.
Chen X, Htet ZM, Lopez-Alfonzo E, Martin A, Walters KJ. Proteasome interaction with ubiquitinated substrates: from mechanisms to therapies. FEBS J. 2021;288:5231–51.
Martinez-Ferriz A, Ferrando A, Fathinajafabadi A, Farras R. Ubiquitin-mediated mechanisms of translational control. Semin Cell Dev Biol. 2022;132:146–54.
Liu M, Yan M, Lv H, Wang B, Lv X, Zhang H, et al. Macrophage K63-linked ubiquitination of YAP promotes its nuclear localization and exacerbates atherosclerosis. Cell Rep. 2020;32:107990.
Wang P, Song J, Ye D. CRL3s: the BTB-CUL3-RING E3 ubiquitin ligases. Adv Exp Med Biol. 2020;1217:211–23.
Baek K, Scott DC, Schulman BA. NEDD8 and ubiquitin ligation by cullin-RING E3 ligases. Curr Opin Struct Biol. 2021;67:101–9.
Kim JE, Lee DS, Kim TH, Park H, Kim MJ, Kang TC. PLPP/CIN-mediated Mdm2 dephosphorylation increases seizure susceptibility via abrogating PSD95 ubiquitination. Exp Neurol. 2020;331:113383.
Zhu J, Lee KY, Jong TT, Tsai NP. C2-lacking isoform of Nedd4-2 regulates excitatory synaptic strength through GluA1 ubiquitination-independent mechanisms. J Neurochem. 2019;151:289–300.
Kim JE, Lee DS, Kim MJ, Kang TC. PLPP/CIN-mediated NEDD4-2 S448 dephosphorylation regulates neuronal excitability via GluA1 ubiquitination. Cell Death Dis. 2019;10:545.
Reynolds JP, Jimenez-Mateos EM, Cao L, Bian F, Alves M, Miller-Delaney SF, et al. Proteomic analysis after status epilepticus identifies UCHL1 as protective against hippocampal injury. Neurochem Res. 2017;42:2033–54.
Smaldone G, Pirone L, Pedone E, Marlovits T, Vitagliano L, Ciccarelli L. The BTB domains of the potassium channel tetramerization domain proteins prevalently assume pentameric states. Febs Lett. 2016;590:1663–71.
Pinkas DM, Sanvitale CE, Bufton JC, Sorrell FJ, Solcan N, Chalk R, et al. Structural complexity in the KCTD family of Cullin3-dependent E3 ubiquitin ligases. Biochem J. 2017;474:3747–61.
Teng X, Aouacheria A, Lionnard L, Metz KA, Soane L, Kamiya A, et al. KCTD: a new gene family involved in neurodevelopmental and neuropsychiatric disorders. CNS Neurosci Ther. 2019;25:887–902.
Alevy J, Burger CA, Albrecht NE, Jiang D, Samuel MA. Progressive myoclonic epilepsy-associated gene Kctd7 regulates retinal neurovascular patterning and function. Neurochem Int. 2019;129:104486.
Metz KA, Teng X, Coppens I, Lamb HM, Wagner BE, Rosenfeld JA, et al. KCTD7 deficiency defines a distinct neurodegenerative disorder with a conserved autophagy-lysosome defect. Ann Neurol. 2018;84:766–80.
Van Bogaert P. KCTD7-related progressive myoclonus epilepsy. Epileptic Disord. 2016;18:115–9.
Lin GN, Corominas R, Lemmens I, Yang X, Tavernier J, Hill DE, et al. Spatiotemporal 16p11.2 protein network implicates cortical late mid-fetal brain development and KCTD13-Cul3-RhoA pathway in psychiatric diseases. Neuron. 2015;85:742–54.
Escamilla CO, Filonova I, Walker AK, Xuan ZX, Holehonnur R, Espinosa F, et al. Kctd13 deletion reduces synaptic transmission via increased RhoA. Nature. 2017;551:227–31.
Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, et al. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell. 2009;35:841–55.
Madison JM, Duong K, Vieux EF, Udeshi ND, Iqbal S, Requadt E, et al. Regulation of purine metabolism connects KCTD13 to a metabolic disorder with autistic features. iScience. 2021;24:101935.
Kusenda M, Vacic V, Malhotra D, Rodgers L, Pavon K, Meth J, et al. The influence of microdeletions and microduplications of 16p11.2 on global transcription profiles. J Child Neurol. 2015;30:1947–53.
Golzio C, Willer J, Talkowski ME, Oh EC, Taniguchi Y, Jacquemont S, et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature. 2012;485:363–7.
Rein B, Yan Z. 16p11.2 copy number variations and neurodevelopmental disorders. Trends Neurosci. 2020;43:886–901.
Dai J, Patzke C, Liakath-Ali K, Seigneur E, Sudhof TC. GluD1 is a signal transduction device disguised as an ionotropic receptor. Nature. 2021;595:261–5.
Xu C, Liu HJ, Qi L, Tao CL, Wang YJ, Shen Z, et al. Structure and plasticity of silent synapses in developing hippocampal neurons visualized by super-resolution imaging. Cell Discov. 2020;6:8.
Xu Y, Song R, Chen W, Strong K, Shrey D, Gedela S, et al. Recurrent seizure-related GRIN1 variant: molecular mechanism and targeted therapy. Ann Clin Transl Neurol. 2021;8:1480–94.
Tang S, Terzic B, Wang IJ, Sarmiento N, Sizov K, Cui Y, et al. Altered NMDAR signaling underlies autistic-like features in mouse models of CDKL5 deficiency disorder. Nat Commun. 2019;10:2655.
Morandell J, Schwarz LA, Basilico B, Tasciyan S, Dimchev G, Nicolas A, et al. Cul3 regulates cytoskeleton protein homeostasis and cell migration during a critical window of brain development. Nat Commun. 2021;12:3058.
Zhu J, Lee KY, Jewett KA, Man HY, Chung HJ, Tsai NP. Epilepsy-associated gene Nedd4-2 mediates neuronal activity and seizure susceptibility through AMPA receptors. PLoS Genet. 2017;13:e1006634.
Judson MC, Wallace ML, Sidorov MS, Burette AC, Gu B, van Woerden GM, et al. GABAergic neuron-specific loss of Ube3a causes angelman syndrome-like EEG abnormalities and enhances seizure susceptibility. Neuron. 2016;90:56–69.
Fang M, Li Y, Ren J, Hu R, Gao X, Chen L. Epilepsy-associated UBE3A deficiency downregulates retinoic acid signalling pathway. Front Genet. 2021;12:681295.
Arbogast T, Razaz P, Ellegood J, McKinstry SU, Erdin S, Currall B, et al. Kctd13-deficient mice display short-term memory impairment and sex-dependent genetic interactions. Hum Mol Genet. 2019;28:1474–86.
Martin Lorenzo S, Nalesso V, Chevalier C, Birling MC, Herault Y. Targeting the RHOA pathway improves learning and memory in adult Kctd13 and 16p11.2 deletion mouse models. Mol Autism. 2021;12:1.
Conboy K, Henshall DC, Brennan GP. Epigenetic principles underlying epileptogenesis and epilepsy syndromes. Neurobiol Dis. 2020;148:105179.
Chen TS, Lai MC, Huang HI, Wu SN, Huang CW. Immunity, ion channels and epilepsy. Int J Mol Sci. 2022;23:6446.
Zhou MH, Chen SR, Wang L, Huang Y, Deng M, Zhang J, et al. Protein kinase C-mediated phosphorylation and alpha2delta-1 interdependently regulate NMDA receptor trafficking and activity. J Neurosci. 2021;41:6415–29.
Wang YQ, Huang YH, Balakrishnan S, Liu L, Wang YT, Nestler EJ, et al. AMPA and NMDA receptor trafficking at cocaine-generated synapses. J Neurosci. 2021;41:1996–2011.
Zhang H, Bramham CR. Bidirectional dysregulation of AMPA receptor-mediated synaptic transmission and plasticity in brain disorders. Front Synaptic Neurosci. 2020;12:26.
Zhou C, Lippman Bell JJ, Sun H, Jensen FE. Hypoxia-induced neonatal seizures diminish silent synapses and long-term potentiation in hippocampal CA1 neurons. J Neurosci. 2011;31:18211–22.
Lippman-Bell JJ, Zhou C, Sun H, Feske JS, Jensen FE. Early-life seizures alter synaptic calcium-permeable AMPA receptor function and plasticity. Mol Cell Neurosci. 2016;76:11–20.
Itoh M, Yamashita M, Kaneko M, Okuno H, Abe M, Yamazaki M, et al. Deficiency of AMPAR-palmitoylation aggravates seizure susceptibility. J Neurosci. 2018;38:10220–35.
Marwick KFM, Hansen KB, Skehel PA, Hardingham GE, Wyllie DJA. Functional assessment of triheteromeric NMDA receptors containing a human variant associated with epilepsy. J Physiol. 2019;597:1691–704.
Soares C, Lee KF. A prominent role for triheteromeric GluN1/GluN2A/GluN2B NMDARs at central synapses. J Neurosci. 2013;33:14975–7.
Li X, Yang KB, Chen W, Mai J, Wu XQ, Sun T, et al. CUL3 (cullin 3)-mediated ubiquitination and degradation of BECN1 (beclin 1) inhibit autophagy and promote tumor progression. Autophagy. 2021;17:4323–40.
Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–6.
Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21.
Clements L, Harvey J. Activation of oestrogen receptor α induces a novel form of LTP at hippocampal temporoammonic‐CA1 synapses. Br J Pharmacol. 2020;177:642–55.
Glasgow SD, Labrecque S, Beamish IV, Aufmkolk S, Gibon J, Han D, et al. Activity-dependent Netrin-1 secretion drives synaptic insertion of GluA1-containing AMPA receptors in the hippocampus. Cell Rep. 2018;25:168–82.e6.
Wong JM, Folorunso OO, Barragan EV, Berciu C, Harvey TL, Coyle JT, et al. Postsynaptic serine racemase regulates NMDA receptor function. J Neurosci. 2020;40:9564–75.
Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94.
Acknowledgements
We express our gratitude to the patients for the donations of brain tissue and their time and effort devoted to the consent process.
Funding
This work was supported by National Natural Science Foundation of China (Nos. 81922023, 82271496, 81873788, 82001378 and 82171440), Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0747 and cstc2021ycjh-bgzxm0035). Future Medical Youth Innovation Team Program of Chongqing Medical University (No. W0043), the Fifth Senior Medical Talents Program of Chongqing for Young and Middle-aged, Middle-aged Medical Excellence Team Program of Chongqing, and Chongqing chief expert studio project, China. Science and Technology Research Program of Chongqing Education Commission (KJQN202200435), Chongqing Talents: Exceptional Young Talents Project (CQYC202005014).
Author information
Authors and Affiliations
Contributions
JG, FX, PK and XW designed the study; JG, PK, HG, JL, YL, XT, XX and DX performed the experiments; DX and YM participated in the data and sample collection; JG, PK, HG and ZH analyzed data; JG, FX and ZH wrote the manuscript; XW and FX oversaw the project. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were approved by the Ethics Committee of Chongqing Medical University. Human brain tissue samples from patients with TLE or TBI were collected from the First Affiliated Hospital of Chongqing Medical University. Written informed consent was obtained from patients for the use of brain tissue and access to medical records for research purposes. The collection and use of all specimens were approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University and in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, J., Ke, P., Guo, H. et al. KCTD13-mediated ubiquitination and degradation of GluN1 regulates excitatory synaptic transmission and seizure susceptibility. Cell Death Differ 30, 1726–1741 (2023). https://doi.org/10.1038/s41418-023-01174-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01174-5