Abstract
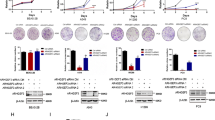
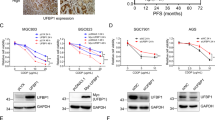
Cell migration and invasion are two important steps for tumour metastasis, and involved the behaviors including metabolism remodeling and anti-apoptosis. However, it’s still elusive that cancer cells how to antagonize apoptosis during tumour metastasis. In this study, we observed that super elongation complex (SEC) subunit AF9 depletion exacerbated cell migration and invasion but reduced the apoptosis during invasive migration. Mechanically, AF9 targeted acetyl (Ac)-STAT6 at lysine (K) 284 and blocked STAT6 transactivation on the promoter of such genes involved in regulating purine metabolism and metastasis, in turn induced apoptosis of suspended cells. Of note, AcSTAT6-K284 was not induced by IL4 signaling but decreased by limited nutrition which triggered SIRT6 to remove acetyl group at STAT6-K284. The functional experiments proved that AcSTAT6-K284 attenuated cell migration and invasion depending on AF9 expression level. Animal metastatic study further confirmed the AF9/AcSTAT6-K284 axis existed and blocked kidney renal clear cell carcinoma (KIRC) metastasis. In clinical, both AF9 expression and AcSTAT6-K284 were decreased accompanied by the advanced tumour grade and positively correlated with KIRC patients’ survival. Conclusively, we explored an inhibitory axis which not only suppressed tumour metastasis but also could be utilized for drug development to hamper KIRC metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
We provided data accession codes for RNA-sequencing original data in the NCBI BioProject as follows: PRJNA874431. Original western blot was uploaded as Supplementary Material.
References
Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Disco. 2022;12:31–46.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201–18.
Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–91.
Wiel C, Le Gal K, Ibrahim MX, Jahangir CA, Kashif M, Yao H, et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019;178:330–45.e22.
Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21:162–80.
Nabi S, Kessler ER, Bernard B, Flaig TW, Lam ET. Renal cell carcinoma: a review of biology and pathophysiology. F1000Res. 2018;7:307.
Chowdhury N, Drake CG. Kidney cancer: an overview of current therapeutic approaches. Urol Clin North Am. 2020;47:419–31.
van de Merbel AF, van der Horst G, van der Pluijm G. Patient-derived tumour models for personalized therapeutics in urological cancers. Nat Rev Urol. 2021;18:33–45.
Sanchez-Gastaldo A, Kempf E, Gonzalez Del Alba A, Duran I. Systemic treatment of renal cell cancer: a comprehensive review. Cancer Treat Rev. 2017;60:77–89.
Tian X, Yu H, Li D, Jin G, Dai S, Gong P, et al. The miR-5694/AF9/Snail axis provides metastatic advantages and a therapeutic target in basal-like breast cancer. Mol Ther. 2021;29:1239–57.
Yu H, He J, Liu W, Feng S, Gao L, Xu Y, et al. The transcriptional coactivator, ALL1-fused gene from chromosome 9, simultaneously sustains hypoxia tolerance and metabolic advantages in liver cancer. Hepatology. 2021;74:1952–70.
Le Masson I, Yu DY, Jensen K, Chevalier A, Courbeyrette R, Boulard Y, et al. Yaf9, a novel NuA4 histone acetyltransferase subunit, is required for the cellular response to spindle stress in yeast. Mol Cell Biol. 2003;23:6086–102.
Luo Z, Lin C, Shilatifard A. The super elongation complex (SEC) family in transcriptional control. Nat Rev Mol Cell Biol. 2012;13:543–7.
Li Y, Wen H, Xi Y, Tanaka K, Wang H, Peng D, et al. AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell. 2014;159:558–71.
Wang CG, Ye YJ, Yuan J, Liu FF, Zhang H, Wang S. EZH2 and STAT6 expression profiles are correlated with colorectal cancer stage and prognosis. World J Gastroenterol. 2010;16:2421–7.
Ostrand-Rosenberg S, Grusby MJ, Clements VK. Cutting edge: STAT6-deficient mice have enhanced tumour immunity to primary and metastatic mammary carcinoma. J Immunol. 2000;165:6015–9.
Verhoeven Y, Tilborghs S, Jacobs J, De Waele J, Quatannens D, Deben C, et al. The potential and controversy of targeting STAT family members in cancer. Semin Cancer Biol. 2020;60:41–56.
Li BH, Yang XZ, Li PD, Yuan Q, Liu XH, Yuan J, et al. IL-4/Stat6 activities correlate with apoptosis and metastasis in colon cancer cells. Biochem Biophys Res Commun. 2008;369:554–60.
Webb DJ, Zhang H, Horwitz AF. Cell migration: an overview. Methods Mol Biol. 2005;294:3–11.
Yamada KM, Sixt M. Mechanisms of 3D cell migration. Nat Rev Mol Cell Biol. 2019;20:738–52.
Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Disco. 2014;13:673–91.
Neumann H, Peak-Chew SY, Chin JW. Genetically encoding N(epsilon)-acetyllysine in recombinant proteins. Nat Chem Biol. 2008;4:232–4.
Zheng Y, Liu Q, Shen H, Yang G. To increase the incorporation efficiency of genetically encoding N(epsilon)-acetyllysine in recombinant protein. Protein Expr Purif. 2018;145:59–63.
Pan PW, Feldman JL, Devries MK, Dong A, Edwards AM, Denu JM. Structure and biochemical functions of SIRT6. J Biol Chem. 2011;286:14575–87.
Lu X, Chen J, Sasmono RT, Hsi ED, Sarosiek KA, Tiganis T, et al. T-cell protein tyrosine phosphatase, distinctively expressed in activated-B-cell-like diffuse large B-cell lymphomas, is the nuclear phosphatase of STAT6. Mol Cell Biol. 2007;27:2166–79.
Eckerling A, Ricon-Becker I, Sorski L, Sandbank E, Ben-Eliyahu S. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer. 2021;21:767–85.
Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–35.
Murugan AK. mTOR: Role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019;59:92–111.
Wang TH, Wang HS, Soong YK. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer. 2000;88:2619–28.
Guo M, Liu Z, Si J, Zhang J, Zhao J, Guo Z, et al. Cediranib induces apoptosis, G1 phase cell cycle arrest, and autophagy in non-small-cell lung cancer cell A549 in vitro. Biomed Res Int. 2021;2021:5582648.
Tasselli L, Zheng W, Chua KF. SIRT6: novel mechanisms and links to aging and disease. Trends Endocrinol Metab. 2017;28:168–85.
Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–29.
Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–30.
Fiorentino F, Carafa V, Favale G, Altucci L, Mai A, Rotili D. The two-faced role of SIRT6 in cancer. Cancers (Basel). 2021;13:1156.
Garcia-Jimenez C, Goding CR. Starvation and pseudo-starvation as drivers of cancer metastasis through translation reprogramming. Cell Metab. 2019;29:254–67.
Rapp UR, Korn C, Ceteci F, Karreman C, Luetkenhaus K, Serafin V, et al. MYC is a metastasis gene for non-small-cell lung cancer. PLoS One. 2009;4:e6029.
Pezzuto A, Carico E. Role of HIF-1 in cancer progression: novel insights. a review. Curr Mol Med. 2018;18:343–51.
Karpathiou G, Papoudou-Bai A, Ferrand E, Dumollard JM, Peoc’h M. STAT6: a review of a signaling pathway implicated in various diseases with a special emphasis in its usefulness in pathology. Pathol Res Pr. 2021;223:153477.
Pfeifer CR, Xia Y, Zhu K, Liu D, Irianto J, Garcia VMM, et al. Constricted migration increases DNA damage and independently represses cell cycle. Mol Biol Cell. 2018;29:1948–62.
Pfeifer CR, Vashisth M, Xia Y, Discher DE. Nuclear failure, DNA damage, and cell cycle disruption after migration through small pores: a brief review. Essays Biochem. 2019;63:569–77.
Hunakova L, Bies J, Sedlak J, Duraj J, Jakubikova J, Takacsova X, et al. Differential sensitivity of ovarian carcinoma cell lines to apoptosis induced by the IMPDH inhibitor benzamide riboside. Neoplasma. 2000;47:274–9.
Arpaia E, Benveniste P, Di Cristofano A, Gu Y, Dalal I, Kelly S, et al. Mitochondrial basis for immune deficiency. Evidence from purine nucleoside phosphorylase-deficient mice. J Exp Med. 2000;191:2197–208.
Swann PF, Waters TR, Moulton DC, Xu YZ, Zheng Q, Edwards M, et al. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996;273:1109–11.
Bahreyni A, Khazaei M, Rajabian M, Ryzhikov M, Avan A, Hassanian SM. Therapeutic potency of pharmacological adenosine receptor agonist/antagonist in angiogenesis, current status and perspectives. J Pharm Pharm. 2018;70:191–6.
Attar R, Panah T, Romero MA, Yulaevna IM, Gazouli M, Berardi R, et al. Overview of the signaling pathways involved in metastasis: an intriguing story-tale of the metastatic journey of ovarian cancer cells. Cell Mol Biol (Noisy-le-Gd). 2021;67:212–23.
Weiss F, Lauffenburger D, Friedl P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat Rev Cancer. 2022;22:157–73.
Carey MF, Peterson CL, Smale ST. Chromatin immunoprecipitation (ChIP). Cold Spring Harb Protoc 2009;2009:pdb prot5279.
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90.
Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–36.
Acknowledgements
The authors thank the CAS Shanghai Institute of Nutrition and Health (SINH) Molecular and metabolism core facility for the metabolite determination and protein synthesis service. This study was supported in part by NSFC grants No. 92053113 and 81570607, National Natural Science Foundation for Young Scholars of China (No. 81902566 and 82103511), and Shanghai Jiaotong University Medical-Engineering Cross Research Fund (No. YG2019QNA53).
Author information
Authors and Affiliations
Contributions
SJ, ST, DY and YH designed, performed, and analyzed experiments, prepared the figures. CL provided technical help. WXJ and FN conceived this study, wrote the manuscript and YH rewrote the revised manuscript. WXJ and WX designed and analyzed experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All KIRC samples were conducted in accordance with the requirements of Shanghai First People’s Hospital, Shanghai Jiaotong University. The use of clinical samples was approved by the institutional review board of the hospital with the ethical number 2021KSQ367.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, J., Shi, T., Chen, L. et al. AF9 targets acetyl-modified STAT6 to diminish purine metabolism and accelerate cell apoptosis during metastasis. Cell Death Differ 30, 1695–1709 (2023). https://doi.org/10.1038/s41418-023-01172-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01172-7
This article is cited by
-
zDHHC3-mediated S-palmitoylation of SLC9A2 regulates apoptosis in kidney clear cell carcinoma
Journal of Cancer Research and Clinical Oncology (2024)