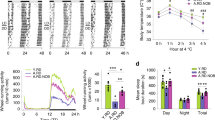
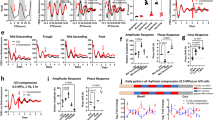
Abstract
Exposure to artificial light at night (LAN) can induce obesity, depressive disorder and osteoporosis, but the pernicious effects of excessive LAN exposure on tissue structure are poorly understood. Here, we demonstrated that artificial LAN can impair developmental growth plate cartilage extracellular matrix (ECM) formation and cause endoplasmic reticulum (ER) dilation, which in turn compromises bone formation. Excessive LAN exposure induces downregulation of the core circadian clock protein BMAL1, which leads to collagen accumulation in the ER. Further investigations suggest that BMAL1 is the direct transcriptional activator of prolyl 4-hydroxylase subunit alpha 1 (P4ha1) in chondrocytes, which orchestrates collagen prolyl hydroxylation and secretion. BMAL1 downregulation induced by LAN markedly inhibits proline hydroxylation and transport of collagen from ER to golgi, thereby inducing ER stress in chondrocytes. Restoration of BMAL1/P4HA1 signaling can effectively rescue the dysregulation of cartilage formation within the developmental growth plate induced by artificial LAN exposure. In summary, our investigations suggested that LAN is a significant risk factor in bone growth and development, and a proposed novel strategy targeting enhancement of BMAL1-mediated collagen hydroxylation could be a potential therapeutic approach to facilitate bone growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting the results are available from the corresponding author upon request.
References
Falchi F, Cinzano P, Duriscoe D, Kyba CC, Elvidge CD, Baugh K, et al. The new world atlas of artificial night sky brightness. Sci Adv. 2016;2:e1600377.
Münzel T, Hahad O, Daiber A. The dark side of nocturnal light pollution. Outdoor light at night increases risk of coronary heart disease. Eur Heart J. 2021;42:831–4.
Navara KJ, Nelson RJ. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res. 2007;43:215–24.
An K, Zhao H, Miao Y, Xu Q, Li YF, Ma YQ, et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat Neurosci. 2020;23:869–80.
Mason IC, Grimaldi D, Reid KJ, Warlick CD, Malkani RG, Abbott SM, et al. Light exposure during sleep impairs cardiometabolic function. Proc Natl Acad Sci USA. 2022;119:e2113290119.
Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014;35:648–70.
Tähkämö L, Partonen T, Pesonen AK. Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int. 2019;36:151–70.
Haraguchi S, Kamata M, Tokita T, Tashiro KI, Sato M, Nozaki M, et al. Light-at-night exposure affects brain development through pineal allopregnanolone-dependent mechanisms. eLife. 2019;8:e45306.
Bedrosian TA, Galan A, Vaughn CA, Weil ZM, Nelson RJ. Light at night alters daily patterns of cortisol and clock proteins in female Siberian hamsters. J Neuroendocrinol. 2013;25:590–6.
Feskanich D, Hankinson SE, Schernhammer ES. Nightshift work and fracture risk: the Nurses’ Health Study. Osteoporos Int. 2009;20:537–42.
Panda S. Circadian physiology of metabolism. Science. 2016;354:1008–15.
Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49.
Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–7.
Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307.
Liu Z, Gan L, Luo D, Sun C. Melatonin promotes circadian rhythm-induced proliferation through Clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J Pineal Res. 2017;62:e12383.
So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA. 2009;106:17582–7.
Schilperoort M, Bravenboer N, Lim J, Mletzko K, Busse B, van Ruijven L, et al. Circadian disruption by shifting the light-dark cycle negatively affects bone health in mice. FASEB J. 2020;34:1052–64.
Yuan G, Hua B, Yang Y, Xu L, Cai T, Sun N, et al. The circadian gene clock regulates bone formation via PDIA3. J Bone Miner Res. 2017;32:861–71.
Yu S, Tang Q, Chen G, Lu X, Yin Y, Xie M, et al. Circadian rhythm modulates endochondral bone formation via MTR1/AMPKβ1/BMAL1 signaling axis. Cell Death Differ. 2022;29:874–87.
Yu S, Tang Q, Xie M, Zhou X, Long Y, Xie Y, et al. Circadian BMAL1 regulates mandibular condyle development by hedgehog pathway. Cell Prolif. 2020;53:e12727.
Tam SKE, Brown LA, Wilson TS, Tir S, Fisk AS, Pothecary CA, et al. Dim light in the evening causes coordinated realignment of circadian rhythms, sleep, and short-term memory. Proc Natl Acad Sci USA. 2021;118:e2101591118.
Dudek M, Gossan N, Yang N, Im HJ, Ruckshanthi JP, Yoshitane H, et al. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J Clin Investig. 2016;126:365–76.
Pihlajaniemi T, Myllylä R, Kivirikko KI. Prolyl 4-hydroxylase and its role in collagen synthesis. J Hepatol. 1991;13 Suppl 3:S2–7.
Huang N, Chelliah Y, Shan Y, Taylor CA, Yoo SH, Partch C, et al. Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science. 2012;337:189–94.
Li Y, Cheng S, Li L, Zhao Y, Shen W, Sun X. Light-exposure at night impairs mouse ovary development via cell apoptosis and DNA damage. Biosci Rep. 2019;39:BSR20181464.
Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96:E463–472.
deHaro D, Kines KJ, Sokolowski M, Dauchy RT, Streva VA, Hill SM, et al. Regulation of L1 expression and retrotransposition by melatonin and its receptor: implications for cancer risk associated with light exposure at night. Nucleic Acids Res. 2014;42:7694–707.
Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–5.
Ma Z, Jin X, Qian Z, Li F, Xu M, Zhang Y, et al. Deletion of clock gene Bmal1 impaired the chondrocyte function due to disruption of the HIF1α-VEGF signaling pathway. Cell Cycle. 2019;18:1473–89.
Chen G, Zhao H, Ma S, Chen L, Wu G, Zhu Y, et al. Circadian rhythm protein Bmal1 modulates cartilage gene expression in temporomandibular joint osteoarthritis via the MAPK/ERK pathway. Front Pharmacol. 2020;11:527744.
Peng P, Wang D, Xu X, Wang D, Gao B, Wang H, et al. Targeting clock-controlled gene Nrf2 ameliorates inflammation-induced intervertebral disc degeneration. Arthritis Res Ther. 2022;24:181.
Abou-Jaoude A, Courtes M, Badique L, Elhaj Mahmoud D, Abboud C, Mlih M, et al. ShcA promotes chondrocyte hypertrophic commitment and osteoarthritis in mice through RunX2 nuclear translocation and YAP1 inactivation. Osteoarthr Cartil. 2022;30:1365–75.
Qian Z, Zhang Y, Kang X, Li H, Zhang Y, Jin X, et al. Postnatal conditional deletion of Bmal1 in osteoblasts enhances trabecular bone formation via increased BMP2 Signals. J Bone Miner Res. 2020;35:1481–93.
Taga Y, Kusubata M, Ogawa-Goto K, Hattori S. Stable isotope-labeled collagen: a novel and versatile tool for quantitative collagen analyses using mass spectrometry. J Proteome Res. 2014;13:3671–8.
Holster T, Pakkanen O, Soininen R, Sormunen R, Nokelainen M, Kivirikko KI, et al. Loss of assembly of the main basement membrane collagen, type IV, but not fibril-forming collagens and embryonic death in collagen prolyl 4-hydroxylase I null mice. J Biol Chem. 2007;282:2512–9.
Zou Y, Donkervoort S, Salo AM, Foley AR, Barnes AM, Hu Y, et al. P4HA1 mutations cause a unique congenital disorder of connective tissue involving tendon, bone, muscle and the eye. Hum Mol Genet. 2017;26:2207–17.
Chang J, Garva R, Pickard A, Yeung CC, Mallikarjun V, Swift J, et al. Circadian control of the secretory pathway maintains collagen homeostasis. Nat Cell Biol. 2020;22:74–86.
Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat Rev Endocrinol. 2014;10:466–75.
Ouyang JQ, Davies S, Dominoni D. Hormonally mediated effects of artificial light at night on behavior and fitness: linking endocrine mechanisms with function. J Exp Biol. 2018;221:jeb156893.
Akagi R, Akatsu Y, Fisch KM, Alvarez-Garcia O, Teramura T, Muramatsu Y, et al. Dysregulated circadian rhythm pathway in human osteoarthritis: NR1D1 and BMAL1 suppression alters TGF-β signaling in chondrocytes. Osteoarthr Cartil. 2017;25:943–51.
Yang W, Kang X, Liu J, Li H, Ma Z, Jin X, et al. Clock gene Bmal1 modulates human cartilage gene expression by crosstalk with Sirt1. Endocrinology. 2016;157:3096–107.
He T, Pang S, Wang H, Yun H, Hao X, Jia L, et al. Drugging the circadian clock feedback cycle to ameliorate cartilage degeneration. FEBS J. 2022;289:6643–58.
Zhang M, Lu Q, Egan B, Zhong XB, Brandt K, Wang J. Epigenetically mediated spontaneous reduction of NFAT1 expression causes imbalanced metabolic activities of articular chondrocytes in aged mice. Osteoarthr Cartil. 2016;24:1274–83.
Stegen S, Laperre K, Eelen G, Rinaldi G, Fraisl P, Torrekens S, et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565:511–5.
Xie M, Tang Q, Nie J, Zhang C, Zhou X, Yu S, et al. BMAL1-downregulation aggravates porphyromonas gingivalis-induced atherosclerosis by encouraging oxidative stress. Circ Res. 2020;126:e15–e29.
Acknowledgements
We would like to thank Prof. Anbing Shi (Huazhong University of Science and Technology) for constructive advice. We also thanks Prof. Ying Xu (Soochow University) for the Bmal1wt/− and Bmal1fl/fl mating pairs.
Funding
This research was funded by the National Natural Science Foundation of China for Key Program Projects (82030070, to LC) and General Program (82270950, to QT), Hubei Provincial Natural Science Fund for Creative Research Group (2020CFA014, to LC), Young Talent Program by Health Commission of Hubei Province (WJ2021Q059, to QT) and the Youth Clinical Research Fund of Chinese Stomatological Association (CSA-O2020-10, to QT).
Author information
Authors and Affiliations
Contributions
GC, QT and LC performed the experiments, data analysis and drafted the manuscript. SY, JS and YS contributed to the materials and analysis methods. GF, GM, YZ, YY, JP and XL contributed to animal housing and animal experiments. QW and LZ supervised the mathematical aspects of this research. LC supervised the whole study and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All experiments were approved by the Institutional Animal Care and Use Committee of Tongji Medical College (LAUCU Number:2809).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, G., Tang, Q., Yu, S. et al. Developmental growth plate cartilage formation suppressed by artificial light at night via inhibiting BMAL1-driven collagen hydroxylation. Cell Death Differ 30, 1503–1516 (2023). https://doi.org/10.1038/s41418-023-01152-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01152-x