Abstract
Mixed lineage kinase domain-like protein (MLKL) emerged as executioner of necroptosis, a RIPK3-dependent form of regulated necrosis. Cell death evasion is one of the hallmarks of cancer. Besides apoptosis, some cancers suppress necroptosis-associated mechanisms by for example epigenetic silencing of RIPK3 expression. Conversely, necroptosis-elicited inflammation by cancer cells can fuel tumor growth. Recently, necroptosis-independent functions of MLKL were unraveled in receptor internalization, ligand-receptor degradation, endosomal trafficking, extracellular vesicle formation, autophagy, nuclear functions, axon repair, neutrophil extracellular trap (NET) formation, and inflammasome regulation. Little is known about the precise role of MLKL in cancer and whether some of these functions are involved in cancer development and metastasis. Here, we discuss current knowledge and controversies on MLKL, its structure, necroptosis-independent functions, expression, mutations, and its potential role as a pro- or anti-cancerous factor. Analysis of MLKL expression patterns reveals that MLKL is upregulated by type I/II interferon, conditions of inflammation, and tissue injury. Overall, MLKL may affect cancer development and metastasis through necroptosis-dependent and -independent functions.
Similar content being viewed by others
Facts
-
MLKL is the executioner of necroptosis, a RIPK3-dependent process. Necroptosis may function as a tumor-suppressing mechanism and as a back-up cell death modality in case of apoptosis resistance.
-
Necroptosis provokes inflammation that can fuel tumor growth.
-
Some cancers suppress necroptosis by epigenetic silencing of RIPK3. MLKL expression is variable in cancer, but no epigenetic silencing mechanisms have been reported for MLKL to date.
-
Little is known about the regulation and exact role of MLKL in cancer development and metastasis. Subcellular functions of MLKL such as receptor/ligand degradation and endosomal trafficking may operate independently of RIPK3.
Open questions
-
Do necroptosis-independent functions of MLKL involve RIPK3-mediated phosphorylation? Which other kinases or mechanisms may activate MLKL?
-
How is MLKL expression regulated in cancer?
-
Does the role of MLKL in cancer involve its cell death-dependent or -independent mechanisms?
-
Is there any evidence of functional MLKL mutations in cancer?
Introduction
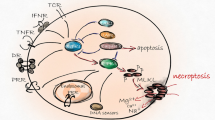
Necroptosis, a form of regulated necrosis, is initiated by the kinase activity of the receptor-interacting protein kinase 3 (RIPK3) in conjunction with one of the other RHIM-containing proteins such as RIPK1, TRIF, or ZBP1, pending on the stimulus during infection and inflammation (reviewed in [1]) (Fig. 1). The mixed lineage kinase domain-like (MLKL) pseudokinase was identified as a downstream target of RIPK3 and final effector of necroptosis execution [2, 3]. Which RHIM-domain-containing protein will recruit and activate RIPK3, depends on the type of signaling receptor that is triggered. The canonical necroptosis pathway is initiated by TNF receptor family ligands (e.g. TNF, TRAIL, TL1A) and requires RIPK1 kinase activity, followed by recruitment and activation of RIPK3, which in its turn activates MLKL by serine-dependent phosphorylation. Various stimuli such as death receptor ligands (e.g. TNF), pathogen-associated molecular patterns (PAMPs) (e.g. LPS) and interferon (IFN) induce necroptosis by different pathways involving distinct RHIM-domain-containing proteins such as RIPK1, TRIF, and ZBP1, that all converge on RIPK3-mediated activation of the single known executioner of necroptosis, viz. MLKL (Fig. 1). RIPK3-dependent phosphorylation of MLKL results in a conformational switch and translocation of MLKL from the cytosol to diverse cellular membranes, including the plasma membrane where it causes loss of membrane integrity and eventually necrotic death. RIPK3 and MLKL constitute the core necroptosis machinery, whereas RIPK1 possesses a dual function as an essential survival factor through its scaffold function and as being a mediator of necroptosis (as well as certain types of apoptosis) through its kinase activity [4, 5]. Whether RIPK1 kinase activity mediates apoptosis or necroptosis depends on the inactivation of caspase-8 activity, a negative regulator of necroptosis [6, 7]. Necroptosis is implicated in a plethora of diseases ranging from degenerative diseases to inflammatory diseases [1] due to its lytic nature of cell death that provokes inflammation [8]. Its possible involvement in cancer is a topic of intense research and discussion [9,10,11]. Necroptosis in cancer can be regarded as a double-edged sword. Discovery of necroptosis as a back-up cell death mechanism gave rise to the concept to therapeutically target necroptosis in cancer cells that acquired apoptosis resistance [9]. Necroptosis, on the other hand, as an inflammatory/immunogenic mode of cell death can potentially fuel cancer growth and metastasis [12] or enhance antitumor immunity [13,14,15]. RIPK3 as the upstream kinase of MLKL has been studied extensively, but little is known about the exact role of MLKL and its regulation in cancer development and metastasis. Few existing literatures on the role of MLKL in cancer are fragmentary and controversial. MLKL and its role in cancer has been exclusively discussed in the context of necroptosis because of the known link between cell death and cancer. However, more recently other functions of MLKL beyond necroptosis are being reported such as receptor/ligand internalization, endosomal trafficking, EV, and exosome formation [16]. Therefore in this review we focus on these cell death-independent functions of MLKL to gain insight into the pleiotropic nature of this protein. In addition, it is known that cancer cells can suppress necroptosis by gene silencing and loss-of-function mutations of essential necroptotic factors. RIPK3 expression is often silenced by promoter methylation in various cancer cells [17]. MLKL expression is variable in cancer, but there is no literature about epigenetic silencing mechanisms for MLKL. Although expression of both RIPK3 and MLKL is postulated to be a prerequisite to necroptosis susceptibility, little is known about the expression regulation of MLKL. Therefore we performed a global gene expression analysis of MLKL using a curated gene expression database to complement literature. Mutations in the MLKL gene in cancer are also reported but functional consequences are vastly unknown [18]. Finally, in the light of these diverse functions and expression regulation of MLKL, we discuss current knowledge and controversies surrounding MLKL and its possible role in cancer development and metastasis.
RIPK3-dependent necroptosis can be initiated by engagement of RIPK1, TRIF, or ZBP-1 through their respective RHIM domains (indicated as black stripe) upon activation by phosphorylation (asterisks). Various stimuli induce necroptosis by different pathways that all converge to the executioner of necroptosis, the pseudokinase MLKL. RIPK3-dependent phosphorylation (indicated as black dot on MLKL molecule) induces a conformational switch in MLKL and oligomerization that finally results in necroptosis. Necroptosis occurs especially under conditions of caspase-8 inhibition or ablation. See the legend to Fig. 2 for MLKL molecular domains.
Molecular structure and function of MLKL
The MLKL protein has two functional domains: an N-terminal four-helix bundle (4HB) and a C-terminal pseudokinase domain (PsKD), connected by a brace domain consisting of two alpha helices [19] (Fig. 2). The 4HB domain is responsible for the killing activity of MLKL [20,21,22,23,24]. A PsKD lacks kinase activity but plays an important role in cellular signaling acting as a dynamic scaffold and modulator of protein-protein interaction [25]. The PsKD domain of MLKL acts as interface with the kinase domain of RIPK3 in a 1:1 constellation [19, 26]. Once RIPK3 is activated, it phosphorylates the PsKD of MLKL (S345, S347, and T349 in mMLKL and T357/S358 in hMLKL), leading to a conformational change in the PsKD that unlocks the 4HB domain followed by a release of MLKL from the RIPK3 activation platform [20, 27, 28] (Fig. 2). Unlike phosphomimetic mMLKL (S345D) in mouse cells, human phosphomimetic MLKL (T357E/S358D) was reported not to induce cell death in U937 and HT29 cells, suggesting that hMLKL may require additional activation steps than mMLKL beyond the phosphorylation of residues in the PsKD by RIPK3 [29]. However, others have reported that expression of the same human phosphomimetic can induce cell death upon stimulation in human cell lines such as RIPK3-HeLa and HT-29 [2, 24, 30]. The reasons for these discrepancies are currently unclear. Therefore, the question whether there are species differences between mMLKL and hMLKL with regard to its full activation remains unanswered. In addition, RIPK3-dependent activation of MLKL displays remarkable species selectivity as mRIPK3 cannot bind and activate hMLKL [2, 31]. A structural and reconstitution study revealed that distinct PsKD confirmations may account for such selectivity [31]. In the mouse context, a ‘kiss and run’ mechanism of RIPK3-dependent MLKL activation has been proposed where a transient interaction with RIPK3 is sufficient [32], whereas in human cellular systems more stable RIPK3-MLKL preformed complexes between RIPK3 KD and MLKL PsKD have been shown [2, 29, 33]. X-ray and NMR co-structures using a covalently bound inhibitor confirmed the interaction site between the auto-inhibitory brace region and the 4HB domain [34]. Other phosphorylation sites have been identified that fine-tune MLKL killing activity (both enhancing and inhibiting), including T376, S228, S158, S124, and S248 [35, 36] (Fig. 2). Not only phosphorylation, but also other structural changes alter MLKL’s killing activity. For example, mutations in the brace region (D139V) and ATP-binding pocket (K219M, Q343A) are described that result in constitutive mMLKL killing activity [32, 37]. Activated MLKL oligomerizes [20, 24, 29, 38] and interacts via a patch of positively charged residues in the 4HB with negatively charged phosphatidylinositol phosphates (PIPs) in the plasma membrane or with cardiolipin [23, 39]. Besides the crucial role of PIPs in the recruitment to the plasma membrane, inositolphosphate (IP6) binding promotes the active conformation of MLKL by displacing the auto-inhibitory brace region [40]. Additionally, it was shown that phosphatidylinositol transfer protein alpha, a transporter of phosphatidylinositol, facilitates necroptosis by binding the N-terminal domain of MLKL and contributing to oligomerization in cisplatin-triggered cell death in human A549 lung cancer cells [41]. Whether this could be a general mechanism of action needs further investigation. Beside the membrane inserting structures described above, MLKL can also form large disulfide bond-dependent amyloid-like fibers consisting of α-helical structures similar to that of mitochondrial antiviral signaling protein and apoptosis-associated spec-like protein (ASC) [42] unlike RIPK1-RIPK3 amyloid-like fibers that mainly contain β sheet conformations [43].
A The functions of each structural domain of MLKL are indicated. The N-terminal 4-helical bundle domain (4HB) shows homology with the N-terminal HeLo-like domain (HELL) of the fungal protein HELLP according to Hidden Markov Models [128]. The PsKD resembles a bi-lobal protein kinase domain, which binds ATP without hydrolyzing it, thus rendering the pseudokinase domain catalytically inactive [91]. Upon RIPK3-dependent phosphorylation (S345) of MLKL, the self-inhibitory pseudokinase domain (PsKD) of MLKL is released from the 4HB, thereby activating MLKL (pMLKLS345) to induce necroptosis. The brace region contributes to the dynamic nature of the 4HB domain, the conformational change induced after activating phosphorylation of the PsKD and the oligomerization of activated MLKL. Phosphorylations that tune cell death activity of MLKL include inhibitory phosphorylation and activating phosphorylation (indicated in red and green respectively). Impact of the phosphorylation of MLKL on its cell death-independent functions is not known yet. h human site, m murine site. B Next to necroptosis execution, MLKL is also involved in ESCRT (Endosomal Sorting Complexes Required for Transport)-dependent repair of the plasma membrane (which restricts necroptotic cell death), release of extracellular vesicles, endosomal trafficking and receptor recycling, myelin sheath membrane breakdown and axon regeneration after injury, muscle stem cell (MuSC) proliferation after muscle injury, NET formation, inflammasome activation, possible nuclear functions including regulation of endothelial cell adhesion molecules such as ICAM1, VCAM1 and E-selectin through interaction with RNA-binding motif protein 6 (RBM6) and stabilization of mRNA, and inhibition of autophagic flux. TNC: tenascin-C. Black dot: phosphorylation of MLKL. Black arrows: processes that require RIPK3-dependent phosphorylation of MLKL, while red arrows: processes that require RIPK3-independent phosphorylation of MLKL.
Endosomal trafficking and ESCRT-III: detoxifying mechanisms of MLKL
MLKL, beyond its role in necroptosis execution, can serve a paradoxical role by counteracting its own cytotoxicity. This depends on the capacity of MLKL to contribute to proper endosomal trafficking from early to late endosomes and intracellular degradation of ligand/receptor complexes such as TNF/TNFR1 and EGF/EGFR, extracellular vesicle (EV) formation, and endosomal sorting complexes required for transport III (ESCRT-III)-dependent removal of damaged membrane during necroptosis. The endosomal trafficking processes involving MLKL do not require RIPK3 kinase activity and can operate during conditions of cellular homeostasis independent of cell death [30, 44]. RIPK3-dependent phosphorylation further enhances MLKL’s ability to generate and release phospho-MLKL (p-MLKL)-containing EVs, thereby protecting against plasma membrane permeabilization and cell death. Moreover, blocking exosome release by silencing Rab27, increases the intracellular p-MLKL levels and sensitizes cells to necroptotic death [30]. Finally, ESCRT-III, in conjunction with MLKL/p-MLKL, is implicated in the detoxifying mechanism of p-MLKL [44, 45]. Generation of p-MLKL causes a quick Ca2+ influx, followed by local exposure of phosphatidylserine (PS) to the cell surface and ESCRT-III-dependent release of ‘bubbles’ containing leaky or damaged plasma membrane at the site of MLKL/p-MLKL cluster formation. MLKL was not detected in those bubbles by time-lapse confocal microscopy. This process is thus likely to contribute to restore plasma membrane integrity and delays the onset of cell death [44]. This delay would create an additional time window for dying cells or cells bound to die to produce relatively more inflammatory cytokines and chemokines (CXCL1, CXCL10), an important phenomenon to alert host immune system [44, 46]. These chemokines play an important role in cancer progression and metastasis by affecting various immune cells such as CD8+ T cells and myeloid cells [47, 48]. CXCL1 secreted from cancer cells attracts CD11b+Gr1+ myeloid cells that in turn produce S1008/9 proteins, factors that promote cancer cell survival in metastatic sites [49]. Although the CXCL10/CXCR3 axis has an established role in tumor suppression, emerging evidence suggests its role in metastasis [50]. CXCL10 produced by brain astrocytes facilitates brain metastasis of melanoma [51]. Targeting of the CXCL10 receptor CXCR3 in either metastatic breast cancer cells or host compartment decreased metastasis of breast cancer cells [52]. Thus modulation of cell death by ESCRT-III dependent-repair mechanism may not only directly impacts the fate of primary tumor cells but also its complex interaction with the host immunity through paracrine and autocrine mechanisms that ultimately determine cell fate and tumor environment responses.
MLKL inhibits autophagic flux during necroptosis
MLKL can also target intracellular membranes in addition to the plasma membrane. The translocation of RIPK3-dependent activated MLKL (pMLKLS345) to the autophagosomal and/or autophagolysosomal membranes results in disruption of membrane integrity, lysosomal dysfunctioning, and inhibition of autophagic flux in mouse dermal fibroblasts [53]. Increased MLKL expression and activation in the liver of high-fat diet-treated mice contributed to liver injury by inhibiting autophagy [54]. Autophagy plays a complex role in cancer [55,56,57], raising an intriguing possibility that MLKL might act via this pathway. Through lysosomal dysfunction, the necroptotic proteins RIPK1, RIPK3, and MLKL accumulated in neurons after spinal cord injury and potentiated necroptosis [58]. However, in fibroblasts and colorectal cancer cells, the canonical autophagic pathway apparently does not affect necroptotic cell death [53]. Therefore, the causal relationship between autophagy, MLKL and necroptosis may depend on the cell type and still needs further investigation.
MLKL in regeneration
MLKL can also contribute to tissue regeneration after injury. Although RIPK3 has been linked to tissue repair through kinase- and necroptosis-independent mechanisms [59], the mechanisms seem different. MLKL is induced in Schwann cells after axon injury and activated through Ser441 phosphorylation by a yet unidentified kinase different from RIPK3. Activated MLKL then translocates and binds to the sphingolipid sulfatide in the myelin sheath membrane in order to destroy this membrane structure to allow regeneration [16] (Fig. 2). MLKL also contributes significantly to muscle stem cell regeneration after muscle injury through necroptosis induction. Tenascin-C expression is induced in necroptotic myofibers and released in the extracellular stem cell microenvironment upon membrane rupture. This factor activates the EGFR signaling pathway in muscle stem cells, which in its turn facilitates muscle generation [60].
Nuclear functions of MLKL
Recently, a part of the MLKL pool has been observed to translocate from the cytosol to the nucleus following necroptotic stimuli [61]. This translocation occurs in association with RIPK1 and RIPK3 before onset of plasma membrane rupture but is apparently independent of cell death, as necrosulfonamide (an inhibitor of hMLKL [2]) treatment or N-terminal tagging of MLKL, disabling the killer domain of MLKL, has no impact on nuclear translocation. Phosphorylation of MLKL is required to expose the nuclear localization signal (NLS, AA 224–256) in the C-terminal domain. Mutations in the NLS sequence modestly decreased cytotoxicity of MLKL, suggesting nuclear translocation may only partly contribute to cell death. Although this report illustrates that p-MLKL drives translocation of RIPK1 and RIPK3 as a complex to the nucleus, another publication indicated constitutive presence of RIPK1 in both nucleus and cytoplasm, implying only shuttling of RIPK3/MLKL between both compartments. Inhibition of nuclear export by Leptomycin B resulted in RIPK3/MLKL nuclear retention and delayed cell death [62]. Although these papers describe different mechanisms of the nuclear localization of RIPK1 and RIPK3 kinases and MLKL, both papers identified its nuclear shuttling as process partially contributing to necroptosis. The mechanism of how nuclear shuttling of MLKL contributes to necroptosis remains enigmatic. Recent papers describe MLKL as regulator of endothelial cell adhesion molecule expression [63, 64]. MLKL can promote vascular inflammation by directly regulating endothelial cell adhesion molecule expression such as ICAM1, VCAM1, and E-selectin, a process that seems to be independent from RIPK3 though. By directly interacting with RNA-binding motif protein 6 (RBM6), it stabilizes mRNA and enhances the expression of these adhesion molecules, which is necessary for the interaction of the endothelial cell with leukocytes [64]. This process is intimately linked to extravasation of tumor cells and contributes to metastasis [65].
MLKL in immune cells: NLRP3 inflammasome activation and NET formation
MLKL activation in monocytes and bone marrow-derived macrophages (BMDMs) also triggers NLRP3 inflammasome activation and caspase-1-dependent but gasdermin-D-independent IL-1β processing and release besides necroptosis [66, 67]. This process is dependent of RIPK3. In neutrophils MLKL contributes to multiple processes. Necroptosis stimulus-induced neutrophil extracellular traps (NETs), a network of extracellular DNA and microbiocidal proteins, entraps and destroys invading pathogens. This process is dependent on RIPK1, RIPK3, and MLKL, linking necroptosis players with NETosis and bactericidal activity [68]. Accordingly, MLKL deficient mice have more neutrophils in the peripheral blood after Staphylococcus aureus infection and are more susceptible to the infection [68]. Moreover, NETs act as a physical shield to protect tumor cells from T cell or NK cell-mediated cytotoxicity [69].
Analysis of a global gene expression database for MLKL
Expression of both RIPK3 and MLKL are required to induce necroptosis. As available literature on gene expression of MLKL is limited, we examined expression of MLKL in silico using curated gene/protein expression databases comprising thousands of experimental data. Data generated by the human protein atlas project (HPA) reveal highly differential expression of MLKL mRNA in human tissues, whereas the expression levels of RIPK3 mRNA were more comparable (Fig. 3). RIPK3 mRNA levels are the highest in the gastrointestinal tract and the skin, while MLKL mRNA levels are the highest in bone marrow and immune system (Fig. 3). MLKL mRNA is not only differentially expressed in tissues, but also strongly responsive to various stimuli comprising cellular environmental cues. Analysis of publically available transcriptomics data curated by Genevestigator illustrates that several perturbations, both in human and mouse, strongly impacts MLKL mRNA expression level, while this is often not the case for RIPK3 mRNA. In mouse models of infection, inflammation, tissue injury, and a type of cancer driven by inflammation, Mlkl expression is highly inducible (Fig. 4). This induction might be crucial for proper functioning of MLKL.
RIPK3 and MLKL mRNA expression data in human tissues, generated by the human protein atlas project (HPA) (www.proteinatlas.org). Bone marrow and immune system, lung, adipose tissue and gallbladder exhibit the highest MLKL gene expression, while the pancreas, brain, skin, and some muscle tissues reveal low MLKL mRNA levels. Overall, RIPK3 mRNA expression levels are lower than MLKL. Both RIPK3 and MLKL have low RNA expression in brain and pancreas. Additionally, RIPK3 mRNA levels are the highest in the gastrointestinal tract and the skin (5–10 TPM), while MLKL mRNA levels are highest in bone marrow and immune system (20–30 TPM). TPM transcripts per million.
Overview of all perturbations tested in mouse that result in at least a 5-fold change in mMlkl mRNA levels both up- or downregulation. Data were collected from Genevestigator based on the ‘affymetrix mouse genome 430 2.0 array’ platform. The 2.5-fold change, a fold change considered to be low, is indicated by dotted line. Mlkl mRNA expression is highly inducible in mouse models of infection, inflammation, tissue injury, and cancer. Signals that activate innate immune response including pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS, a component of Gram-negative bacteria that activates both TLR4 and inflammasome), infections with parasites, viruses, and bacteria, wound healing as well as chemical-induced inflammation strongly induce Mlkl mRNA expression in various cell types and tissues. Also, IFN signaling induces Mlkl mRNA expression. T. cruzi infection, that activates expression of IFN-stimulated genes through type I IFN receptor (IFNAR1) signaling upregulates Mlkl mRNA [70]. Secondly, IFN-γ-treated BMDMs have increased Mlkl mRNA levels that are even further augmented when treated in combination with LPS, indicating that interferon and TLR/inflammasome signaling operate in synergy to regulate Mlkl mRNA expression. Finally, IFN-regulated transcription factors such as signal transducer and activator of transcription 1 (STAT1), STAT2, and IFN-regulatory factor 9 (IRF9) seem to mediate the induction of Mlkl by IFNα, as respective KOs result in reduced Mlkl mRNA levels. Mice that develop nonalcoholic steatohepatitis (NASH; Gnmt KO mice), have a higher risk to develop hepatocellular carcinoma (HCC). Not only does NASH upregulate Mlkl mRNA expression in the liver, but also HCC further increases its level (up to 7-fold change).
Additionally, both type I interferon (IFN-α/β) and type II IFN (IFN-γ) seem to be a major regulator of Mlkl mRNA during infection, inflammation and cancer (Fig. 4). Trypanosoma cruzi infection, that activates expression of IFN-stimulated genes through type I IFN receptor (IFNAR1) signaling upregulates Mlkl mRNA [70]. IFNγ-treated BMDMs have increased Mlkl levels that are even further augmented when treated in combination with LPS, indicating that IFN and TLR signaling work in synergy to regulate MLKL expression. IFN-regulated transcription factors such as signal transducer and activator of transcription 1 (STAT1), STAT2 and IFN-regulatory factor 9 (IRF9) mediate the induction of Mlkl mRNA by IFNα, as respective gene ablation result in reduced MLKL levels. Also, tissue injury and inflammation-driven cancer upregulate Mlkl mRNA expression. Glycine N-methyltransferase (GNMT) gene deficient mice that develop nonalcoholic steatohepatitis (NASH), have a higher risk to develop hepatocellular carcinoma (HCC). NASH upregulate Mlkl mRNA expression in the liver, that is further augmented in HCC (Fig. 4). On the other hand, immune cells in melanoma tumor tissue have decreased Mlkl mRNA levels compared to splenocytes. Under these conditions that highly regulate Mlkl mRNA expression, Ripk3 mRNA expression is rarely altered. Only upon certain infections (Clostridium difficile and Mycobacterium tuberculosis), inflammation and tissue injury, Ripk3 mRNA levels are moderately increased (Fig. 4). Altogether, these data suggest a highly inducible Mlkl gene versus a Ripk3 gene expression that remains rather unaffected. The highly responsive expression pattern of Mlkl gene may underlie experimental variability found in animal models and account for conflicting reports [71]. RIPK3 gene expression seems to be dominantly regulated by promoter methylation as reported in many cancer cells [17]. This differential regulation of Mlkl and Ripk3 mRNA may reflect their distinct cellular functions independent of necroptosis. Moreover, it is also conceivable that beside the regulation at the gene level, other kinases than RIPK3 may further regulate MLKL at the protein level in various cellular functions, as has been reported in case of striatal injury [16].
MLKL as an interferon-stimulated gene (ISG)
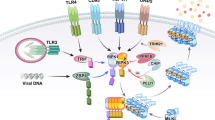
A link between IFN-signaling and necroptosis execution has been established in literature using macrophages and mouse embryonic fibroblast (MEF) cells [72,73,74,75] (Fig. 5). Many stimuli including TLR ligands, IFN-α/β and IFN-α/β-inducing factors result in activation of the IFN-stimulated gene factor 3 complex, consisting of STAT1, STAT2, and IRF9, followed by sustained formation of the necrosome and necroptosis execution. Type I interferon-signaling is important for necroptosis, as Ifnar1 deficiency results in resistance to necroptosis in macrophages stimulated with LPS, poly-I:C, TNF or IFN-β in combination with caspase inhibitors [72, 73]. Irf3/7 KO, Ifnar1 KO, or Stat2 KO BMDMs survive low-dose LPS treatment and have reduced MLKL expression [74, 76]. Pathogens such as S. enterica and S. typhimurium can take advantage of this by eliminating macrophages via IFN-α/β-induced RIPK3-dependent necroptosis [73]. Type I IFNs are also reported to augment the expression of other cell death regulating proteins by an epigenetic mechanism involving acetyltransferases Kat2b and P300 [76] (Fig. 5). Although type I IFN-induced expression of MLKL is required for necroptosis, reconstitution with MLKL in Ifnar1 KO BMDMs could not restore cell death sensitivity, indicating the involvement of other unknown ISGs [18, 77]. Steady-state levels of IFN-β determine expression of ISGs including STAT1/2 and MLKL in macrophages [77]. This steady-state expression of MLKL is important for its oligomerization and necroptosis. On the other hand, Ifn-β-deficient mice display higher p-MLKL levels in the bronchoalveolar lavage fluid during viral asthma exacerbation model [78]. It is likely that the impact of IFN-β may be dependent on cell type.
IFN signaling induces transcription of MLKL by different mechanisms, including direct binding of STAT1 to the promoter of MLKL and IRF-regulated expression of acetyltransferases that can activate transcription of MLKL by acetylation of its promoter. Also CREB can directly bind to the MLKL promoter, thereby repressing MLKL expression. Finally, REST can (in)directly activate MLKL transcription. * = another interferon-stimulated gene that might be involved in necroptosis signaling. Irf1/3/7/9 interferon regulatory factor 1/3/7/9, Gsdmd gasdermin D, Casp 8/11 caspase-8/11, Zbp1 Z-DNA binding protein 1, STAT1/2 signal transducer and activator of transcription 1/2, PKR protein kinase R, ISG interferon-stimulated gene, H3K27Ac histon 3 lysin 27 acetylation, p300 histone acetyltransferase p300, Kat2b lysine acetyltransferase 2b, TRIF TIR-domain-containing adaptor-protein inducing IFN-β, PCB-95 polychlorinated biphenyl-95, LPS lipopolysaccharide, IFNgammaR interferon gamma receptor, IFNAR1 interferon alpha receptor, TLR3/4 toll-like receptor 3/4, REST RE1-silencing transcription factor, CREB cAMP responsive element binding protein, BRD4 bromodomain 4 protein, RNA-POLII RNA polymerase II, P-TEFb positive transcription elongation factor.
Not only type I IFNs but also type II IFNs induce MLKL (but not RIPK3) expression, resulting in necroptosis [79], consistent with the data from Genevestigator in the previous section. Upregulation of MLKL protein has been detected in liver tissues of patients with autoimmune hepatitis and of mice with ConA-induced hepatitis through IFN-γ-dependent activation of STAT1 that directly binds to the MLKL promoter and induces its transcription [80] (Fig. 5).
In contrast to MEF cells and macrophages, IFN-γ downregulates MLKL expression in splenocytes and protects against TNF/zVAD-fmk-induced necroptosis [81]. Also Ifng KO mice with collagen-induced arthritis have increased MLKL protein expression in the synovium [81]. Overall, IFN-γ may have a different impact on MLKL expression levels depending on cell type and disease context. Several cancer cell lines can be sensitized to necroptosis through IFN-γ- and STAT1/IRF1-dependent MLKL upregulation [82, 83]. Intercellular cues from the tumor microenvironment might therefore influence susceptibility of tumor cells to death-inducing stimuli. IFN-γ is secreted by tumor-infiltrating leukocytes such as CD8+ T cells and NK cells and alters tumor microenvironment [84, 85]. The regulation of MLKL by IFN signaling may be highly relevant in cancer biology and treatment options.
Finally, other transcriptional activators of MLKL gene expression have been described, such as the bromodomain protein BRD4. Together with acetylated IRF1, positive transcription elongation factor b (P-TEFb) and RNA polymerase II, BRD4 forms a transcriptional complex that binds to the MLKL promoter region [86]. Bromodomain and extra-terminal domain inhibitors (such as JQ-1) protect against necroptosis by downregulation of MLKL and moderately ameliorate TNF-induced shock in vivo [86]. The neurotoxicant non-dioxin-like polychlorinated biphenyl (PCB)-95 increases Ripk1/3 and Mlkl gene expression and induces necroptosis in cortical neurons [87] (Fig. 5). This induced gene expression depends on both activation of RE1-silencing transcription factor (REST) and downregulation of cAMP responsive element binding protein (CREB).
MLKL expression and mutations in cancer
Deregulation of necroptosis signaling has been observed in many cancer types. In this context, RIPK1 upregulation, RIPK3 and CYLD downregulation, CYLD mutations resulting in truncated forms lacking its functional domain, RIPK1/RIPK3 SNPs and RIPK3 V458M mutations have been described in different cancer types a.o. including non-Hodgkin lymphoma, glioblastoma, melanoma, pancreatic cancer, and hematopoietic cancers [18, 88]. MLKL mutation (L291P, a potential loss of function mutation) or deregulation is observed in many human cancer types, but needs further functional characterization [18]. Depending on the type of cancer, MLKL mRNA levels may differ largely (Fig. 6). A limited selection of human cancers from Genevestigator illustrates highly different transcript levels of MLKL (Fig. 6A). Cancers displaying high levels of MLKL mRNA include colon adenoma and hematopoietic cancers, while brain tumors display very low MLKL mRNA levels. This expression pattern may be reflecting different MLKL mRNA expression in these tissues (Fig. 3). Although MLKL mRNA level is low in skin tissue, different types of skin cancer have variable MLKL mRNA levels ranging from medium to high (Fig. 6A). Similar results are obtained with the few mouse cancer models analyzed to date (Fig. 6B). In COSMIC (catalogue of somatic mutations in cancer) opposing outcomes are described: both up- and downregulation of MLKL mRNA expression, as well as copy number variation gain or loss, are observed over a wide range of cancer types. To date, three MLKL mutations have been investigated in human cancers. Two mutations, found in human gastric cancer, are located at highly conserved residues in the PsKD of human MLKL (F398I and L291P, corresponding with residue F385 and L280 in mMLKL) [32, 89, 90]. The corresponding F385I mMLKL mutant can apparently still induce necroptosis [18, 32], but the impact of this mutation on cell death-independent functions of MLKL, such as endosomal trafficking, EV formation and nuclear localization, have not been reported. On the other hand, the L291P MLKL mutation is likely a loss of function mutation since the corresponding mouse mutant (L280P) cannot induce necroptosis after stimulation [32]. Finally, the MLKL E351K mutation in the non-conventional GFE motif (instead of the DFG motif) at the activation loop of the PsKD has been detected in human lung carcinoma [18, 91]. Although the functional outcome and consequence for disease prognosis is not known, this mutation may alter the affinity for ATP binding [91].
MLKL/Mlkl mRNA expression data of a small selection of human (A) and mouse (B) cancer types. Cancers displaying high levels of MLKL/Mlkl mRNA include colon adenoma and hematopoietic cancers, while brain tumors display very low levels. This expression pattern may be reflecting different MLKL/Mlkl mRNA expression in these tissues (Fig. 2). Although MLKL/Mlkl mRNA level is low in skin tissue, different types of skin cancer have variable MLKL/Mlkl mRNA levels ranging from medium to high (orange). Data were collected from Genevestigator using ‘affymetrix human genome U133 Plus 2.0 Array’ (A) and ‘affymetrix mouse genome 430 2.0 Array’ (B) respectively as platform. Expression levels are indicated according to ‘LOW’, ‘MEDIUM’, and ‘HIGH’, referring to the expression value range determined by looking at all expression values of all genes over all samples for the platform used. LOW = first quartile range, MEDIUM = interquartile range (IQR), and HIGH = fourth quartile.
MLKL as anti-cancerous factor
Low MLKL protein expression is associated with decreased overall survival in patients with resected pancreatic adenocarcinoma, independent from adjuvant chemotherapy [92, 93]. Also in resected colon cancer, gastric cancer, cervical cancer and ovarian cancer similar correlations were observed [94,95,96,97,98]. Additionally, breast cancer and several subtypes of acute myeloid leukemia (AML) have reduced MLKL mRNA expression [18, 99,100,101]. The downregulation of RIPK3 and MLKL in AML suggests the existence of selective forces that propagate AML progression by inducing necroptosis-resistance [100, 102]. A meta-analysis including 613 cancer patients indicated that low MLKL levels are indeed associated with advanced tumor stage and higher lymph node metastasis [103]. Downregulation, loss-of-function mutations or SNPs are found in necroptosis-inducing genes in different types of cancer, suggesting that these factors can limit cancer development and metastasis [18]. Therefore, necroptosis-inducing therapy became an attractive alternative to kill chemotherapy-induced apoptosis-resistant tumors and to elicit immunogenic cell death [14, 104, 105]. Recent strategies include in vivo targeting of tumor cells with Mlkl mRNA through electroporation [105], immune-oncolytic therapy boosting antitumor immunity after tumor-cell specific introduction of Mlkl mRNA [106] or in vivo targeting of tumor cells with liposomes containing Mlkl pDNA together with SMAC mimetic BV6 and zVAD-fmk. These liposomes can reduce substantially the growth of subcutaneous CT26 tumors in syngeneic BALB-c mice [107].
The role of necroptosis mediators goes beyond the control of cancer cells and extends to host immune cells such as tumor associated macrophages (TAM) that influence the tumor growth. MLKL mRNA and protein levels were upregulated in whole blood and peripheral blood mononuclear cells of cervical cancer patients and high MLKL level was associated with better overall survival [96], suggesting prognostic value of MLKL expression levels. Co-culturing U937-derived macrophages with cervical cancer cells results in a decrease in MLKL expression/phosphorylation upon LPS/zVAD-fmk stimulation, decreased necroptotic response and decreased M1 polarization, suggesting that cervical cancer cells apparently affect MLKL expression levels in TAMs to drive a more immunosuppressive environment [96]. Both RIPK3 and MLKL have been implicated in anti-cancer immune responses in vaccination assays in vivo [13, 14, 104, 105, 108]. Lung carcinoma deficient in MLKL or RIPK3 exhibited reduced response to cytostatic chemotherapy by mitoxantrone (MTX) or oxaliplatin (OXA) and revealed reduced anti-cancer immune response upon re-challenge [108]. Additionally, exogenous Mlkl mRNA induces antitumor immunity in melanoma and lymphoma [105]. All these findings are highly suggestive that induction of necroptosis may contribute to vaccination efficiency in these tumor cells. In human breast cancer tissue MLKL mRNA expression levels are positively correlated with immune cell infiltration and local antitumor immune responses by CD8+ T cells and NK cells [101].
MLKL as pro-cancerous factor
In contrast to the concept of necroptosis as backup cell death mechanism to be exploited in anti-cancer therapy, recent evidence suggests that the necroptosis pathway may also contribute to cancer progression in certain conditions. Necroptosis of cancer cells ignites inflammation in the tumor microenvironment that can feed tumor growth. RIPK1/RIPK3-dependent necroptotic signaling induces CXCL1 and SAP130 expression in pancreatic ductal adenocarcinoma in mice, recruiting inhibitory TAMs and tumor-infiltrating myeloid cells respectively, creating an immunosuppressive tumor microenvironment with low T-cell infiltration and consequent tumor progression [109, 110]. Besides RIPK1/3 also MLKL protein expression is increased in patients [109], with increased MLKL levels found at the invasive front of human pancreatic cancer tissue [111]. RIPK1/3 or MLKL gene ablation in the breast cancer cell line MDA-MB-231 resulted in sensitization to radiation, reduced growth in soft agar and delayed tumor growth when injected subcutaneously into nude mice [112]. Decreased overall survival is associated with increased p-MLKL (S358) levels in tumor tissue of esophagus, head and neck squamous cell carcinoma and colon cancer patients and with high MLKL expression in low grade glioma and glioblastoma [112,113,114]. How to explain this paradoxical finding? One may think of the function of MLKL in receptor turnover and endosomal trafficking [30] resulting in increased cancer cell survival or in detoxification of p-MLKL at the level of cancer cells [44, 45] which may contribute to cancer-promoting conditions.
A panel of AML cell lines were reported to express p-RIPK1/3 and p-MLKL (S358) constitutively in contrast to healthy hematopoietic stem/progenitor cells [115]. In these basal conditions, p-MLKL was found in the nucleus and on the centrosomes/spindle of dividing cells, suggesting that at least in some AMLs p-MLKL may execute cell death-independent functions contributing to cancer cell survival and proliferation.
Opposing roles of MLKL in metastasis
The role of MLKL in metastasis is also controversial, as opposing results have been described. Co-culturing human umbilical vein endothelial cells with MDA-MB-231 breast cancer cells induced p-MLKL (S358) activation, while knockdown of MLKL blocked tumor cell-induced endothelial cell necroptosis involving amyloid precursor protein binding to its cognate death receptor 6 [116]. Endothelial cell-specific gene ablation of Ripk3 or Mlkl reduced the number of tumor nodules in the lung, indicating that tumor cell-induced necroptosis of the endothelial cells would promote metastasis by facilitating endothelial fenestration and extravasation of the tumor cells [117]. However, whether necroptosis in endothelial cells is really involved, was questioned in an independent report using similar tumor models. Although the resistance to tumor-induced killing of endothelial cells was reproduced in Ripk3 KO mice, no prominent difference in lung colonization of B16F10 or LLC1 tumor cells was observed in Mlkl KO mice or Ripk3 kinase-dead knockin mice, excluding necroptosis-dependent mechanisms [117]. Later, two other reports also specifically questioned the involvement of endothelial cell necroptosis in this model [118, 119]. On the other hand, MLKL-deficiency in cancer cells resulted in reduced metastasis in an orthotopic breast cancer model [118] and in nasopharyngeal carcinoma lung colonization model [120]. It is suggested that an inflammatory environment due to necroptotic cancer cell death in the primary tumor can drive metastasis [118]. Another report suggests that CXCL5 released by necroptotic cancer cells themselves can trigger neighboring cancer cells to migrate [111]. Chemokines attract distinct sets of immune cells to the tumor microenvironment, which promote metastasis by enhancing cancer cell migration and survival [47]. Release of cytokines by necroptotic cancer cells are important mechanisms to govern tumor-host immunity, and can be modulated by ESCRT-III-dependent repair mechanism [44]. Alternatively, MLKL is suggested to directly contribute to cell migration by activating cell-surface proteases of the disintegrin and metalloprotease (ADAM) family [121] and to a shift in gene expression profile favoring epithelial-to-mesenchymal transition in nasopharyngeal carcinoma [120]. Overall, absence of MLKL in the tumor cells itself or in the tumor microenvironment will influence metastasis differently.
Conclusion and perspectives
MLKL does not only execute necroptosis, but is also involved in receptor turnover, endosomal trafficking, cytokine secretion, EV formation, NET formation, autophagic flux, tissue injury or regeneration and is also found in the nucleus for yet to be identified mechanisms. Several of these necroptosis-independent functions have been linked to cancer progression or metastasis [55,56,57] [122,123,124] [125,126,127]. Whether or not MLKL is involved in these processes needs further investigation. The impact of lack of MLKL protein expression in cancer might be more complex and extend beyond necroptosis resistance. Overall, the pro- or anti-cancerous role of MLKL in carcinogenesis and disease progression, including its regulation by extracellular factors (such as type I and type II IFNs), may depend on the cancer type, timing, and its cellular microenvironment (such as the type of infiltrating immune cells). For example, overexpression of exogenous Mlkl mRNA can result in antitumor immunity in melanoma and lymphoma [105], especially in combination with immune checkpoint blockade, but may also create immune suppressive tumor microenvironments in other tumors as was shown in pancreas cancer [109]. MLKL gene expression levels are very variable depending on the type of cancer (Fig. 6) in contrast to RIPK3 gene that is primarily regulated by epigenetic silencing. MLKL protein deficiency in cancer cells may result in anti- or pro-cancerous conditions, depending on the balance between cancer cell elimination and nature of immune activation by the lytic mode of cell death (inflammatory, immunogenic, immune suppressive depending on the cancer cell types and infiltrate composition). Furthermore, cell death-independent functions of MLKL such as detoxification mechanisms involving inhibition of autophagic flux, modulate cancer cell survival. Distinct expression profiles of RIPK3 and MLKL genes are highly suggestive of such MLKL functions playing a role in cancer in a highly context-dependent manner. Which exact function of MLKL plays a dominant role in a given cancer cell type might determine the outcome. Likewise, MLKL protein deficiency in the cells in the tumor stroma such as TAMs and endothelial cells determine the tumor microenvironment that modulates cancer initiation, growth, and metastasis through multiple processes such as modulation of antitumor immunity and tumor cell extravasation. Here again necroptosis-independent functions of MLKL might play a prominent role. An example of cell death modulation by ESCRT-III-dependent repair mechanism [44] not only directly affects the fate of the primary cancer cells, but also impact the intercellular communication between the cancer cells and the tumor microenvironment. Thus necroptosis and necoptosis-independent functions of MLKL form an intricate network that determines cancer cell survival, growth and metastasis. Better understanding of the complex and opposing functions of MLKL in cancer is needed in order to exploit or target MLKL in anti-cancer therapies. To this end, robust in vivo models such as tissue-specific KOs with well-controlled experimental settings could help to resolve the current controversies in future.
References
Choi ME, Price DR, Ryter SW, Choi AMK. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 2019;4:e128834.
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27.
Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA. 2012;109:5322–7.
Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20.
Delanghe T, Dondelinger Y, Bertrand MJM. RIPK1 kinase-dependent death: a symphony of phosphorylation events. Trends Cell Biol. 2020;30:189–200.
Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–85.
Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, Dohse M, et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019;574:428–31.
Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: toward identification of the effector molecules. Science. 2016;352:aaf2154.
Su Z, Yang Z, Xie L, DeWitt JP, Chen Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016;23:748–56.
Najafov A, Chen H, Yuan J. Necroptosis and cancer. Trends Cancer. 2017;3:294–301.
Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K, et al. The role of necroptosis in cancer biology and therapy. Mol Cancer. 2019;18:100.
Zhu F, Zhang W, Yang T, He SD. Complex roles of necroptosis in cancer. J Zhejiang Univ Sci B. 2019;20:399–413.
Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, et al. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350:328–34.
Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, Heyndrickx L, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15:274–87.
Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17:262–75.
Ying Z, Pan C, Shao T, Liu L, Li L, Guo D, et al. Mixed lineage kinase domain-like protein MLKL breaks down myelin following nerve injury. Mol Cell. 2018;72:457–68 e5.
Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, Koo JS, et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015;25:707–25.
Lalaoui N, Brumatti G. Relevance of necroptosis in cancer. Immunol Cell Biol. 2017;95:137–45.
Petrie EJ, Hildebrand JM, Murphy JM. Insane in the membrane: a structural perspective of MLKL function in necroptosis. Immunol Cell Biol. 2017;95:152–9.
Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci USA. 2014;111:15072–7.
Chen X, Li W, Ren J, Huang D, He WT, Song Y, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–21.
Tanzer MC, Matti I, Hildebrand JM, Young SN, Wardak A, Tripaydonis A, et al. Evolutionary divergence of the necroptosis effector MLKL. Cell Death Differ. 2016;23:1185–97.
Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–81.
Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–46.
Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–52.
Xie T, Peng W, Yan C, Wu J, Gong X, Shi Y. Structural insights into RIP3-mediated necroptotic signaling. Cell Rep. 2013;5:70–8.
Grootjans S, Vanden Berghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017;24:1184–95.
Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016;23:76–88.
Petrie EJ, Sandow JJ, Jacobsen AV, Smith BJ, Griffin MDW, Lucet IS, et al. Conformational switching of the pseudokinase domain promotes human MLKL tetramerization and cell death by necroptosis. Nat Commun. 2018;9:2422.
Yoon S, Kovalenko A, Bogdanov K, Wallach D. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity 2017;47:51–65 e7.
Davies KA, Fitzgibbon C, Young SN, Garnish SE, Yeung W, Coursier D, et al. Distinct pseudokinase domain conformations underlie divergent activation mechanisms among vertebrate MLKL orthologues. Nat Commun. 2020;11:3060.
Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–53.
Petrie EJ, Czabotar PE, Murphy JM. The structural basis of necroptotic cell death signaling. Trends Biochem Sci. 2019;44:53–63.
Rubbelke M, Fiegen D, Bauer M, Binder F, Hamilton J, King J, et al. Locking mixed-lineage kinase domain-like protein in its auto-inhibited state prevents necroptosis. Proc Natl Acad Sci USA. 2020;117:33272–81.
Tanzer MC, Tripaydonis A, Webb AI, Young SN, Varghese LN, Hall C, et al. Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop. Biochem J. 2015;471:255–65.
Najafov A, Mookhtiar AK, Luu HS, Ordureau A, Pan H, Amin PP, et al. TAM kinases promote necroptosis by regulating oligomerization of MLKL. Mol Cell. 2019;75:457–68 e4.
Hildebrand JM, Kauppi M, Majewski IJ, Liu Z, Cox AJ, Miyake S, et al. A missense mutation in the MLKL brace region promotes lethal neonatal inflammation and hematopoietic dysfunction. Nat Commun. 2020;11:3150.
Huang D, Zheng X, Wang ZA, Chen X, He WT, Zhang Y, et al. The MLKL channel in necroptosis is an octamer formed by tetramers in a dyadic process. Mol Cell Biol. 2017;37:e00497–16.
Quarato G, Guy CS, Grace CR, Llambi F, Nourse A, Rodriguez DA, et al. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol Cell. 2016;61:589–601.
Dovey CM, Diep J, Clarke BP, Hale AT, McNamara DE, Guo H, et al. MLKL requires the inositol phosphate code to execute necroptosis. Mol Cell. 2018;70:936–48 e7.
Jing L, Song F, Liu Z, Li J, Wu B, Fu Z, et al. MLKL-PITPalpha signaling-mediated necroptosis contributes to cisplatin-triggered cell death in lung cancer A549 cells. Cancer Lett. 2018;414:136–46.
Liu S, Liu H, Johnston A, Hanna-Addams S, Reynoso E, Xiang Y, et al. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc Natl Acad Sci USA. 2017;114:E7450–E9.
Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–50.
Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell. 2017;169:286–300 e16.
Guo H, Kaiser WJ. ESCRTing necroptosis. Cell. 2017;169:186–7.
van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20:218–32.
Do HTT, Lee CH, Cho J. Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers. 2020;12:287.
Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72:9–18.
Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–78.
Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—a target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–7.
Doron H, Amer M, Ershaid N, Blazquez R, Shani O, Lahav TG, et al. InflammatorY Activation of Astrocytes Facilitates Melanoma Brain Tropism via the CXCL10-CXCR3 signaling axis. Cell Rep. 2019;28:1785–98 e6.
Zhu G, Yan HH, Pang Y, Jian J, Achyut BR, Liang X, et al. CXCR3 as a molecular target in breast cancer metastasis: inhibition of tumor cell migration and promotion of host anti-tumor immunity. Oncotarget. 2015;6:43408–19.
Frank D, Vaux DL, Murphy JM, Vince JE, Lindqvist LM. Activated MLKL attenuates autophagy following its translocation to intracellular membranes. J Cell Sci. 2019;132:jcs220996.
Wu X, Poulsen KL, Sanz-Garcia C, Huang E, McMullen MR, Roychowdhury S, et al. MLKL-dependent signaling regulates autophagic flux in a murine model of non-alcohol-associated fatty liver and steatohepatitis. J Hepatol. 2020;73:616–27.
Santana-Codina N, Mancias JD, Kimmelman AC. The role of autophagy in cancer. Annu Rev Cancer Biol. 2017;1:19–39.
Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci. 2018;19:3466.
Mulcahy Levy JM, Thorburn A. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020;27:843–57.
Liu S, Li Y, Choi HMC, Sarkar C, Koh EY, Wu J, et al. Lysosomal damage after spinal cord injury causes accumulation of RIPK1 and RIPK3 proteins and potentiation of necroptosis. Cell Death Dis. 2018;9:476.
Moriwaki K, Balaji S, Bertin J, Gough PJ, Chan FK. Distinct kinase-independent role of RIPK3 in CD11c(+) mononuclear phagocytes in cytokine-induced tissue repair. Cell Rep. 2017;18:2441–51.
Zhou S, Zhang W, Cai G, Ding Y, Wei C, Li S, et al. Myofiber necroptosis promotes muscle stem cell proliferation via releasing Tenascin-C during regeneration. Cell Res. 2020;30:1063–77.
Yoon S, Bogdanov K, Kovalenko A, Wallach D. Necroptosis is preceded by nuclear translocation of the signaling proteins that induce it. Cell Death Differ. 2016;23:253–60.
Weber K, Roelandt R, Bruggeman I, Estornes Y, Vandenabeele P. Nuclear RIPK3 and MLKL contribute to cytosolic necrosome formation and necroptosis. Commun Biol. 2018;1:6.
Cai F, Wang JL, Wu YL, Hu YW, Wang Q. Mixed lineage kinase domain-like protein promotes human monocyte cell adhesion to human umbilical vein endothelial cells via upregulation of intercellular adhesion molecule-1 expression. Med Sci Monit. 2020;26:e924242.
Dai J, Zhang C, Guo L, He H, Jiang K, Huang Y, et al. A necroptotic-independent function of MLKL in regulating endothelial cell adhesion molecule expression. Cell Death Dis. 2020;11:282.
Janiszewska M, Primi MC, Izard T. Cell adhesion in cancer: beyond the migration of single cells. J Biol Chem. 2020;295:2495–505.
Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Nunez G, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci USA. 2017;114:E961–E9.
Gutierrez KD, Davis MA, Daniels BP, Olsen TM, Ralli-Jain P, Tait SW, et al. MLKL activation triggers NLRP3-mediated processing and release of IL-1beta Independently of gasdermin-D. J Immunol. 2017;198:2156–64.
D’Cruz AA, Speir M, Bliss-Moreau M, Dietrich S, Wang S, Chen AA, et al. The pseudokinase MLKL activates PAD4-dependent NET formation in necroptotic neutrophils. Sci Signal. 2018;11:eaao1716.
Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52:856–71 e8.
Chessler AD, Unnikrishnan M, Bei AK, Daily JP, Burleigh BA. Trypanosoma cruzi triggers an early type I IFN response in vivo at the site of intradermal infection. J Immunol. 2009;182:2288–96.
Alvarez-Diaz S, Preaudet A, Samson AL, Nguyen PM, Fung KY, Garnham AL, et al. Necroptosis is dispensable for the development of inflammation-associated or sporadic colon cancer in mice. Cell Death Differ. 2020. https://doi.org/10.1038/s41418-020-00673-z.
McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, et al. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci USA. 2014;111:E3206–13.
Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–62.
Legarda D, Justus SJ, Ang RL, Rikhi N, Li W, Moran TM, et al. CYLD proteolysis protects macrophages from TNF-mediated auto-necroptosis induced by LPS and licensed by type I IFN. Cell Rep. 2016;15:2449–61.
Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci USA. 2013;110:E3109–18.
Li Y, Guo X, Hu C, Du Y, Guo C, Di W, et al. Type I IFN operates pyroptosis and necroptosis during multidrug-resistant A. baumannii infection. Cell Death Differ. 2018;25:1304–18.
Sarhan J, Liu BC, Muendlein HI, Weindel CG, Smirnova I, Tang AY, et al. Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ. 2019;26:332–47.
Cerps SC, Menzel M, Mahmutovic Persson I, Bjermer L, Akbarshahi H, Uller L. Interferon-beta deficiency at asthma exacerbation promotes MLKL mediated necroptosis. Sci Rep. 2018;8:4248.
Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–202.
Gunther C, He GW, Kremer AE, Murphy JM, Petrie EJ, Amann K, et al. The pseudokinase MLKL mediates programmed hepatocellular necrosis independently of RIPK3 during hepatitis. J Clin Investig. 2016;126:4346–60.
Lee SH, Kwon JY, Kim SY, Jung K, Cho ML. Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis. Sci Rep. 2017;7:10133.
Cekay MJ, Roesler S, Frank T, Knuth AK, Eckhardt I, Fulda S. Smac mimetics and type II interferon synergistically induce necroptosis in various cancer cell lines. Cancer Lett. 2017;410:228–37.
Knuth AK, Rosler S, Schenk B, Kowald L, van Wijk SJL, Fulda S. Interferons transcriptionally up-regulate MLKL expression in cancer cells. Neoplasia. 2019;21:74–81.
Kursunel MA, Esendagli G. The untold story of IFN-gamma in cancer biology. Cytokine Growth Factor Rev. 2016;31:73–81.
Thibaut R, Bost P, Milo I, Cazaux M, Lemaitre F, Garcia Z, et al. Bystander IFN-gamma activity promotes widespread and sustained cytokine signaling altering the tumor microenvironment. Nat Cancer. 2020;1:302–14.
Xiong Y, Li L, Zhang L, Cui Y, Wu C, Li H, et al. The bromodomain protein BRD4 positively regulates necroptosis via modulating MLKL expression. Cell Death Differ. 2019;26:1929–41.
Guida N, Laudati G, Serani A, Mascolo L, Molinaro P, Montuori P, et al. The neurotoxicant PCB-95 by increasing the neuronal transcriptional repressor REST down-regulates caspase-8 and increases Ripk1, Ripk3 and MLKL expression determining necroptotic neuronal death. Biochem Pharm. 2017;142:229–41.
Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17:25–34.
Forbes SA, Beare D, Bindal N, Bamford S, Ward S, Cole CG, et al. COSMIC: high-resolution cancer genetics using the catalogue of somatic mutations in cancer. Curr Protoc Hum Genet. 2016;91:10 1 1–1 37.
Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–D7.
Murphy JM, Lucet IS, Hildebrand JM, Tanzer MC, Young SN, Sharma P, et al. Insights into the evolution of divergent nucleotide-binding mechanisms among pseudokinases revealed by crystal structures of human and mouse MLKL. Biochem J. 2014;457:369–77.
Colbert LE, Fisher SB, Hardy CW, Hall WA, Saka B, Shelton JW, et al. Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer 2013;119:3148–55.
Seldon CS, Colbert LE, Hall WA, Fisher SB, Yu DS, Landry JC. Chromodomain-helicase-DNA binding protein 5, 7 and pronecrotic mixed lineage kinase domain-like protein serve as potential prognostic biomarkers in patients with resected pancreatic adenocarcinomas. World J Gastrointest Oncol. 2016;8:358–65.
Li X, Guo J, Ding AP, Qi WW, Zhang PH, Lv J, et al. Association of mixed lineage kinase domain-like protein expression with prognosis in patients with colon cancer. Technol Cancer Res Treat. 2017;16:428–34.
Ertao Z, Jianhui C, Kang W, Zhijun Y, Hui W, Chuangqi C, et al. Prognostic value of mixed lineage kinase domain-like protein expression in the survival of patients with gastric caner. Tumour Biol. 2016;37:13679–85.
Li L, Yu S, Zang C. Low necroptosis process predicts poor treatment outcome of human papillomavirus positive cervical cancers by decreasing tumor-associated macrophages M1 polarization. Gynecol Obstet Investig. 2018;83:259–67.
Ruan J, Mei L, Zhu Q, Shi G, Wang H. Mixed lineage kinase domain-like protein is a prognostic biomarker for cervical squamous cell cancer. Int J Clin Exp Pathol. 2015;8:15035–8.
He L, Peng K, Liu Y, Xiong J, Zhu FF. Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. Onco Targets Ther. 2013;6:1539–43.
Hockendorf U, Yabal M, Jost PJ. Killing AML: RIPK3 leads the way. Cell Cycle. 2017;16:3–4.
Hockendorf U, Yabal M, Herold T, Munkhbaatar E, Rott S, Jilg S, et al. RIPK3 restricts myeloid leukemogenesis by promoting cell death and differentiation of leukemia initiating cells. Cancer Cell. 2016;30:75–91.
Stoll G, Ma Y, Yang H, Kepp O, Zitvogel L, Kroemer G. Pro-necrotic molecules impact local immunosurveillance in human breast cancer. Oncoimmunology. 2017;6:e1299302.
Nugues AL, El Bouazzati H, Hetuin D, Berthon C, Loyens A, Bertrand E, et al. RIP3 is downregulated in human myeloid leukemia cells and modulates apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis. 2014;5:e1384.
Hu B, Shi D, Lv X, Chen S, Huang Q, Xie M, et al. Prognostic and clinicopathological significance of MLKL expression in cancer patients: a meta-analysis. BMC Cancer. 2018;18:736.
Aaes TL, Verschuere H, Kaczmarek A, Heyndrickx L, Wiernicki B, Delrue I, et al. Immunodominant AH1 antigen-deficient necroptotic, but not apoptotic, murine cancer cells induce antitumor protection. J Immunol. 2020;204:775–87.
Van Hoecke L, Van Lint S, Roose K, Van Parys A, Vandenabeele P, Grooten J, et al. Treatment with mRNA coding for the necroptosis mediator MLKL induces antitumor immunity directed against neo-epitopes. Nat Commun. 2018;9:3417.
Van Hoecke L, Riederer S, Saelens X, Sutter G, Rojas JJ. Recombinant viruses delivering the necroptosis mediator MLKL induce a potent antitumor immunity in mice. Oncoimmunology. 2020;9:1802968.
Sun D, Zhao L, Lin J, Zhao Y, Zheng Y. Cationic liposome co-encapsulation of SMAC mimetic and zVAD using a novel lipid bilayer fusion loaded with MLKL-pDNA for tumour inhibition in vivo. J Drug Target. 2018;26:45–54.
Yang H, Ma Y, Chen G, Zhou H, Yamazaki T, Klein C, et al. Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology. 2016;5:e1149673.
Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532:245–9.
Seifert L, Miller G. Molecular pathways: the necrosome-a target for cancer therapy. Clin Cancer Res. 2017;23:1132–6.
Ando Y, Ohuchida K, Otsubo Y, Kibe S, Takesue S, Abe T, et al. Necroptosis in pancreatic cancer promotes cancer cell migration and invasion by release of CXCL5. PLoS One. 2020;15:e0228015.
Liu XJ, Zhou M, Mei LY, Ruan JY, Hu Q, Peng J, et al. Key roles of necroptotic factors in promoting tumor growth. Oncotarget. 2016;7:22219–33.
Dong Y, Sun Y, Huang Y, Dwarakanath B, Kong L, Lu JJ. Upregulated necroptosis-pathway-associated genes are unfavorable prognostic markers in low-grade glioma and glioblastoma multiforme. Transl Cancer Res. 2019;8:821–7.
Li J, Huang S, Zeng L, Li K, Yang L, Gao S, et al. Necroptosis in head and neck squamous cell carcinoma: characterization of clinicopathological relevance and in vitro cell model. Cell Death Dis. 2020;11:391.
Xin J, You D, Breslin P, Li J, Zhang J, Wei W, et al. Sensitizing acute myeloid leukemia cells to induced differentiation by inhibiting the RIP1/RIP3 pathway. Leukemia. 2017;31:1154–65.
Strilic B, Yang L, Albarran-Juarez J, Wachsmuth L, Han K, Muller UC, et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536:215–8.
Hanggi K, Vasilikos L, Valls AF, Yerbes R, Knop J, Spilgies LM, et al. RIPK1/RIPK3 promotes vascular permeability to allow tumor cell extravasation independent of its necroptotic function. Cell Death Dis. 2017;8:e2588.
Jiao D, Cai Z, Choksi S, Ma D, Choe M, Kwon HJ, et al. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018;28:868–70.
Patel S, Webster JD, Varfolomeev E, Kwon YC, Cheng JH, Zhang J, et al. RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ. 2020;27:161–75.
Dong Y, Sun Y, Huang Y, Fang X, Sun P, Dwarakanath B, et al. Depletion of MLKL inhibits invasion of radioresistant nasopharyngeal carcinoma cells by suppressing epithelial-mesenchymal transition. Ann Transl Med. 2019;7:741.
Cai Z, Zhang A, Choksi S, Li W, Li T, Zhang XM, et al. Activation of cell-surface proteases promotes necroptosis, inflammation and cell migration. Cell Res. 2016;26:886–900.
Han L, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18:59.
Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18:55.
Sheehan C, D’Souza-Schorey C. Tumor-derived extracellular vesicles: molecular parcels that enable regulation of the immune response in cancer. J Cell Sci. 2019;132:jcs235085.
Snoderly HT, Boone BA, Bennewitz MF. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 2019;21:145.
Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227.
Flemming A. Tumours use NETs as physical shields. Nat Rev Drug Discov. 2020;19:388.
Johnston A, Wang Z. Necroptosis: MLKL polymerization. J Nat Sci. 2018;4:e513.
Acknowledgements
SM and JB obtained a PhD fellowship from the IWT (now FWO-SB). SM, JB, NT were paid by Methusalem. Research in the Vandenabeele group is supported by Flemish grants (EOS MODEL-IDI, FWO Grant 30826052), FWO research grants (G.0E04.16N, G.0C76.18N, G.0B71.18N, G.0B96.20N), Methusalem (BOF16/MET_V/007), iBOF20/IBF/039 ATLANTIS, Foundation against Cancer (FAF-F/2016/865, F/2020/1505), CRIG and GIGG consortia, and VIB.
Funding
Funding for this work is mentioned in the acknowledgements.
Author information
Authors and Affiliations
Contributions
SM: concept, design of figures, performing analysis, writing. JB: correction of text and figures. RR: performing analysis, figures. PV: concept, design of figures, writing and correction of text. NT: concept, design of figures, writing and correction of text
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics statement
This review did not require ethical approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by F. Pentimalli
Rights and permissions
About this article
Cite this article
Martens, S., Bridelance, J., Roelandt, R. et al. MLKL in cancer: more than a necroptosis regulator. Cell Death Differ 28, 1757–1772 (2021). https://doi.org/10.1038/s41418-021-00785-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-021-00785-0
This article is cited by
-
Mediators of necroptosis: from cell death to metabolic regulation
EMBO Molecular Medicine (2024)
-
Erythronecroptosis: an overview of necroptosis or programmed necrosis in red blood cells
Molecular and Cellular Biochemistry (2024)
-
Identification and assessment of differentially expressed necroptosis long non-coding RNAs associated with periodontitis in human
BMC Oral Health (2023)
-
MLKL post-translational modifications: road signs to infection, inflammation and unknown destinations
Cell Death & Differentiation (2023)
-
A bibliometric analysis of ferroptosis, necroptosis, pyroptosis, and cuproptosis in cancer from 2012 to 2022
Cell Death Discovery (2023)