Abstract
Introduction
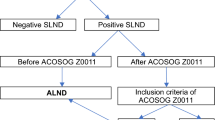
The MonarchE trial explored the use of abemaciclib, a CDK4/6 inhibitor, as an adjuvant treatment in high-risk early-stage luminal-like breast cancer. The study’s inclusion criteria, especially the N2 status, may require revisiting surgical interventions, including invasive axillary lymph node dissection (ALND)—a procedure that current guidelines generally do not recommend.
Methods
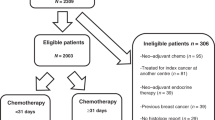
We conducted a single-centre, retrospective, observational cohort study on non-metastatic breast cancer patients managed from 2002 to 2011, at the Institut Curie. Data collection involved clinical and histological characteristics plus treatment follow-up.
Results
Out of 8715 treated patients, 721 met the inclusion criteria. Overall, 12% (87) were classified as N2 ( ≥ 4 positive lymph nodes), thus eligible for abemaciclib per “node criterion.” Tumour size, positive sentinel lymph nodes, and lobular histology showed a significant correlation with N2 status. Approximately 1000 ALNDs would be required to identify 120 N2 cases and prevent four recurrences.
Conclusion
The MonarchE trial may significantly affect surgical practices due to the need for invasive procedures to identify high-risk patients for adjuvant abemaciclib treatment. The prospect of unnecessary morbidity demands less invasive N2 status determination methods. Surgical decisions must consider patient health and potential treatment benefits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Not applicable.
References
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol J Eur Soc Med Oncol. 2011;22:1736–47.
Cardoso F, Spence D, Mertz S, Corneliussen-James D, Sabelko K, Gralow J, et al. Global analysis of advanced/metastatic breast cancer: decade report (2005-2015). Breast Edinb Scotl. 2018;39:131–8.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52.
Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6:116–24.
Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol J Am Soc Clin Oncol. 2017;35:2875–84.
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol J Am Soc Clin Oncol. 2017;35:3638–46.
Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5.
Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38:3987–98.
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. J Am Med Assoc. 2011;305:569–75.
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of Axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. J Am Med Assoc. 2017;318:918–26.
Donker, Tienhoven M, van G, Straver, Meijnen ME, Velde P, van de CJH, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–10.
Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. 2020;18:452–78.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33.
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. 2022;20:691–722.
Geyer CE, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol J Eur Soc Med Oncol. 2022;33:1250–68.
Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst. 2006;98:599–609.
Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol J Am Soc Clin Oncol. 2005;23:4312–21.
Hack TF, Cohen L, Katz J, Robson LS, Goss P. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol J Am Soc Clin Oncol. 1999;17:143–9.
Burak WE, Hollenbeck ST, Zervos EE, Hock KL, Kemp LC, Young DC. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg. 2002;183:23–7.
Sávolt Á, Péley G, Polgár C, Udvarhelyi N, Rubovszky G, Kovács E, et al. Eight-year follow up result of the OTOASOR trial: the optimal treatment of the axilla—surgery or radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: a randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2017;43:672–9.
Katz A, Smith BL, Golshan M, Niemierko A, Kobayashi W, Raad RA, et al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol J Am Soc Clin Oncol. 2008;26:2093–8.
Pilewskie M, Mautner SK, Stempel M, Eaton A, Morrow M. Does a positive axillary lymph node needle biopsy result predict the need for an axillary lymph node dissection in clinically node-negative breast cancer patients in the ACOSOG Z0011 era? Ann Surg Oncol. 2016;23:1123–8.
Davidson T, Shehade N, Nissan E, Sklair-Levy M, Ben-Haim S, Barshack I, et al. PET/CT in breast cancer staging is useful for evaluation of axillary lymph node and distant metastases. Surg Oncol. 2021;38:101567.
Veronesi U, De Cicco C, Galimberti VE, Fernandez JR, Rotmensz N, Viale G, et al. A comparative study on the value of FDG-PET and sentinel node biopsy to identify occult axillary metastases. Ann Oncol. 2007;18:473–8.
Cooper KL, Harnan S, Meng Y, Ward SE, Fitzgerald P, Papaioannou D, et al. Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol EJSO. 2011;37:187–98.
Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. New Engl J Med. 2016;375:717–29.
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. New Engl J Med. 2018;379:111–21.
Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. New Engl J Med. 2021;385:2336–47.
Slamon DJ, Stroyakovskiy D, Yardley DA, Huang CS, Fasching PA, Crown J, et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: primary results from the phase III NATALEE trial. J Clin Oncol. 2023;41:LBA500–LBA500.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualisation: TG and EL. Methodology: TG and EL. Formal analysis: TG and EL. Writing—original draft: TG. Writing review and editing: TG, JP, J-GF, NG, LP, EG, LD, BG, J-YP, A-SH-P, FR and EL. Supervision: EL. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Institut Curie and conducted in accordance with institutional and ethical rules regarding research on tissue samples and patients. All patients gave their initial consent to the use of their anonymized data.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gaillard, T., Piketty, J., Feron, JG. et al. Rethinking surgical revisions: impact of the MonarchE trial on axillary dissection in hormone-positive HER2-negative early breast cancer patients potentially eligible for abemaciclib. Br J Cancer 130, 1141–1148 (2024). https://doi.org/10.1038/s41416-024-02580-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-024-02580-3