Abstract
Background
Whether cancer-related fatigue develops differently after curative-intended oesophageal cancer treatment and the related modifiable factors are unclear.
Methods
This population-based and longitudinal cohort included 409 oesophageal cancer patients who underwent curative oesophagectomy in 2013–2020 in Sweden. The main outcome was cancer-related fatigue trajectories with measurements at 1, 1.5, 2, 2.5, 3, 4 and 5 years postoperatively by validated EORTC QLQ-FA12 questionnaire, and analysed using growth mixture models. Weighted logistic regressions provided odds ratios (OR) with 95% confidence intervals (95% CI) for underlying sociodemographic, clinical, and patient-reported outcome factors in relation to the identified trajectories.
Results
Two distinct overall cancer-related fatigue trajectories were identified: low level of persistent fatigue and high level of increasing fatigue, with 64% and 36% of patients, respectively. The odds of having high level of fatigue trajectory were increased by Charlson comorbidity index (≥ 2 versus 0: OR = 2.52, 95% CI 1.07–5.94), pathological tumour Stage (III–IV versus 0-I: OR = 2.52, 95% CI 1.33–4.77), anxiety (OR = 7.58, 95% CI 2.20–26.17), depression (OR = 15.90, 95% CI 4.44–56.93) and pain (continuous score: OR = 1.02, 95% CI 1.01–1.04).
Conclusions
Long-term trajectories with high level of increasing cancer-related fatigue and the associated modifiable factors were identified after oesophageal cancer treatment. The results may facilitate early identification and targeted intervention for such high-risk patients.
Similar content being viewed by others
Introduction
Oesophageal cancer is one of the leading causes of cancer incidence and mortality globally [1]. Surgery is the mainstay of curatively intended treatment, usually combined with neoadjuvant therapy for locally advanced oesophageal cancer [2]. The treatment is challenging and extensive, often followed by diminished health-related quality of life (HRQL) [2]. The 5-year overall survival for oesophageal cancer patients is <20% worldwide [3,4,5], and the 5-year survival reaches 30–50% after the curative-intent oesophagectomy [6,7,8]. With the prolonged survival, the accompanying long-term survivorship is of increasing importance to clarify.
Cancer-related fatigue refers to the distressing, subjective feeling of tiredness related to cancer or cancer treatment, that is not proportional to recent activity and interferes with daily-life functioning. Cancer-related fatigue is a multidimensional symptom, comprising physical, emotional, and cognitive components [9, 10]. It is one of the most common, severe and persistent HRQL-related symptoms in oesophageal cancer patients [11,12,13]. The aetiology of cancer-related fatigue is rather vague and evidence suggests a multifactorial process involving demographic, physiological, medical, psychosocial, and behavioural factors [14, 15]. Longitudinal research on cancer-related fatigue in oesophageal cancer survivors is scarce and classic approaches focusing on the average population level may conceal the heterogeneity in cancer-related fatigue development over time.
This study aims to explore the potentially distinct trajectories of cancer-related fatigue among oesophageal cancer survivors until five years after oesophagectomy and the potential factors associated with the long-term trajectories. Such evidence could assist healthcare professionals, patients, and family caregivers with a better understanding and preparation for cancer-related fatigue, contribute to the early identification of high-risk groups, and facilitate prompt interventions and targeted follow-up.
Methods
Study design
This is an ongoing Swedish nationwide and prospective cohort study entitled “Oesophageal Surgery on Cancer patients-Adaptation and Recovery (OSCAR) study” [11, 16]. The cohort enrolled all patients with oesophageal cancer who underwent oesophagectomy between January 1, 2013, and June 30, 2020, in Sweden. OSCAR follows up the patients regularly from 1 to 12 years after surgery. For the purpose of this study, all available data until June 30, 2022, i.e., up to 5 years after oesophagectomy was used, despite some patients having more than 5-year follow-up. The project was approved by the Regional Ethical Review Board in Stockholm (2013/844-31/1). Informed consent forms were obtained from all participants.
Data source and collection
Patients were identified via pathology departments at all 8 hospitals performing surgery for oesophageal cancer in Sweden, and those who were alive 1 year after oesophagectomy were included. Cancer-related fatigue was reported by patients at 1, 1.5, 2, 2.5, 3, 4 and 5 years postoperatively. At 1 and 5 years, a research nurse visited the patients and conducted personal interviews using computer-based questionnaires, and patients responded to mailed questionnaires during other follow-ups. During the 1-year follow-up, patient-reported information was collected on anxiety, depression, pain, insomnia, physical activity, height and average weight as an adult. Vital status was retrieved from the National Register of the Total Population with deterministic ascertainment by patient’s unique identification number. Age, sex and education information were obtained from the longitudinal integrated database for health insurance and labour market studies (LISA) using patient’s unique identification number. To estimate the proxy baseline fatigue level (before diagnosis) for the oesophageal cancer patients, 6969 random samples from the Swedish population (reference population) were selected, 4910 (70.5%) of whom participated and 4867 (99.1%) responded to the same questionnaire (EORTC QLQ-C30 fatigue subscale) as patients [17]. Each patient in OSCAR was individually matched to more than 50 persons from the reference population by age at surgery (5-year time window), sex, education level (< 9, 9–12 or >12 years of formal education), and comorbidities (cardiovascular, respiratory, diabetes and others). The proxy baseline fatigue level for each oesophageal cancer patient was calculated as the mean score of cancer-related fatigue from the matched individuals. Clinical data, including comorbidity, tumour histology, treatment, pathological tumour stage, postoperative complications and weight at the time of operation were collected by a review of medical records (histopathology reports, operation charts and discharge notes) according to a predefined protocol.
Outcomes
Cancer-related fatigue was the study outcome, measured by two validated questionnaires developed by the European Organization for Research and Treatment of Cancer (EORTC): Fatigue subscale of the EORTC Quality of Life Core Questionnaire 30 (EORTC QLQ-C30) and the EORTC QLQ-Fatigue 12 (EORTC QLQ-FA12) [18, 19]. The official Swedish translation versions of the questionnaires, with consistent translation and cultural equivalence of the measures, were used in this study [20]. The 30-item EORTC QLQ-C30 questionnaire evaluates HRQL in cancer patients, including a three-item subscale measuring cancer-related fatigue. EORTC QLQ-FA12 is a 12-item multidimensional questionnaire, which is supplemented with the EORTC QLQ-C30 in specific detail. QLQ-FA12 incorporates three multi-item scales measuring physical, emotional, and cognitive aspects of cancer-related fatigue, and two single items to assess interference with daily life (whether the tiredness interferes with patients’ daily activities) and social sequelae (whether the tiredness is not understood by the people who are close to patients). The primary outcomes were cancer-related fatigue trajectories (categorical variable) based on QLQ-C30 fatigue and QLQ-FA12 overall fatigue. The secondary outcomes were trajectories (categorical variable) based on the 3 symptom scales and 2 single items in QLQ-FA12. Fatigue questionnaire responses were transformed into 0-100 scores. Higher scores indicate more cancer-related fatigue. Missing data were handled in line with the EORTC scoring manual [21].
Underlying factors
Nine predefined sociodemographic and clinical factors that were considered to potentially influence the cancer-related fatigue trajectory were included: Age at surgery (continuous variable), sex (male or female), education level (< 9, 9–12 or >12 years of formal education), proxy baseline QLQ-C30 fatigue score (continuous variable), comorbidity (Charlson comorbidity index 0, 1 or ≥2, excluding oesophageal cancer) [22], tumour histology (squamous cell carcinoma or adenocarcinoma), neoadjuvant chemo(radio)therapy (no or yes), pathological tumour Stage (0-I, II or III–IV), and 30-day postoperative complications (Clavien–Dindo classifications 0–I, II–IIIa or IIIb–IV) [23].
Six additional patient-reported outcome factors were also included: anxiety (Hospital Anxiety and Depression Scale [HADS] anxiety score ≥8 was regarded as clinically relevant anxiety symptom: no or yes) [24], depression (HADS depression score ≥8 was regarded as clinically relevant depression symptom: no or yes), pain score (EORTC QLQ-C30, continuous variable), insomnia score (EORTC QLQ-C30, continuous variable), preoperative body mass index (BMI) adjusted weight loss grading system (categorised by BMI at operation and weight loss between average weight as an adult and at the time of operation: 0, 1, 2, 3 or 4) [25], and physical activity one year after oesophagectomy (International Physical Activity Questionnaire [IPAQ]: low, moderate, and high level) [26]. In particular, pain and insomnia were analysed as a continuous score, so their effect size was calculated by each score increase and was expected to be small (a statistically significant small OR might still indicate meaningful associations). These factors were selected based on existing literature [10, 12, 27,28,29,30,31,32,33,34] and availability in the study cohort.
Statistical analysis
Growth mixture models were used to identify separable unobserved (latent) trajectories of cancer-related fatigue, a latent-class analysing method that could identify subgroups following similar longitudinal courses of the outcome within a heterogeneous population [35,36,37,38]. First, the model was fitted with a single linear trajectory. Trajectory numbers were then gradually increased until the model reached the best-identified performance. Up to four trajectories, different latent or residual variances, and trajectory shapes (linear or quadratic) were fitted and compared for each outcome. Model performance was assessed by a combination of the following criteria: Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), sample-size adjusted BIC, entropy, Vuong–Lo–Mendell–Rubin test (VLMR), adjusted Lo–Mendell–Rubin test (aLMR), and model interpretability [39]. BIC, sample-size adjusted BIC, and AIC compare the log-likelihood of nested models, and smaller values imply better model fit. Entropy represents the trajectory classification uncertainty, where higher values indicate better accuracy. VLMR and aLMR compare the K-trajectory with the (K-1)-trajectory models and a significant P value (≤0.05) favours the fit of the K-trajectory model. Models with reasonable interpretation and better statistical index were selected. Models with a trajectory size of less than 15% of the patients were rejected. The posterior probabilities of trajectory membership for each patient were estimated by the selected models, and patients were assigned to the trajectory with the highest posterior probabilities, i.e., the most likely trajectory for the patient. The model-estimated mean and sample mean for each trajectory were output from the growth mixture models. The sample mean was calculated as the mean of fatigue scores with the posterior probabilities in each trajectory as weights. Patients with at least one cancer-related fatigue measurement were included, and all available data were used in the growth mixture models, assuming that data are missing at random.
Weighted logistic regression models calculated odds ratios (OR) with 95% confidence intervals (CI) to estimate associations between underlying factors and cancer-related fatigue trajectories. The reference outcome trajectory was the trajectory with the lowest level of cancer-related fatigue. The selected factors were included in multivariable models and assumed to be confounders for each other. Factors were included step by step: sociodemographic and clinical factors were first included, and then patient-reported outcome factors (anxiety, depression, pain and insomnia) were also added to the models. Due to major missing in the factors preoperative body mass index (BMI) adjusted weight loss grading system and IPAQ physical activity, these two were added to the model in the last step. The weights used were the posterior probabilities of the assigned trajectories from growth mixture models. Statistical significance was tested at two-sided 5% levels. Sensitivity analyses were conducted regarding QLQ-C30 fatigue and QLQ-FA12 overall fatigue, excluding patients who had only one fatigue measurement. Growth mixture models were analysed by MPlus version 8.7 software (Los Angeles, California: Muthén & Muthén), and all other analyses were done using SAS version 9.4 software (Cary, North Carolina: SAS Institute Inc.).
Results
Patients
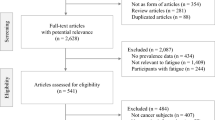
Among the 1013 oesophageal cancer patients who underwent oesophagectomy between January 1, 2013, and June 30, 2020, in Sweden, 242 (23.9%) died within one year after surgery, and 154 (15.2%) were unreachable, leaving 617 eligible patients for study inclusion. Of these, 143 (23.2%) declined to consent, 30 (4.9%) were too sick to participate, 26 (4.2%) had tumour recurrence, and therefore 418 (67.7%) patients participated in the study. Cancer-related fatigue measurements were missing in 9 of the participating patients, and the final study cohort incorporated 409 patients who responded to at least one of the postoperative QLQ-C30 fatigue measurements. At 1 year after oesophagectomy, 404 patients completed the questionnaire, followed by 314 (77.7%), 277 (88.2%), 209 (75.5%), 186 (89.0%), 125 (67.2%), 78 (62.4%) at 1.5, 2, 2.5, 3, 4 and 5 years. The respective attrition numbers for each fatigue measurement are also shown in Figs. 1 and 2.
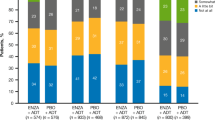
(1) Solid lines represent estimated means. The dotted lines represent sample means. (2) The percentage after each trajectory is the final patient proportion for the trajectory category based on the most likely trajectory membership. (3) Grey dashed lines represent thresholds for clinical importance (Giesinger et al. [40]; Friedrich et al. [41]).
The mean age of the participants was 67.2 years, and the majority were men (91.7%), had a tumour of adenocarcinoma histology (86.1%), and underwent neoadjuvant Chemo(radio)therapy (80.4%) (Supplementary Table S1).
Cancer-related fatigue trajectories
Two similar distinct trajectories were identified for the primary outcomes of QLQ-C30 fatigue and QLQ-FA12 overall fatigue: 64% of the patients experienced a persistently low level of fatigue, while 36% experienced a higher level of fatigue which seemed to increase with time (Fig. 1). The trajectories of high-level fatigue regarding QLQ-C30 and QLQ-FA12 overall measurements were above the threshold for clinical importance (Fig. 1) [40, 41]. As for the specific aspects of cancer-related fatigue (Fig. 2), two trajectories were identified for physical fatigue: persistently low levels of symptoms and a higher level of symptoms increase with time. Two trajectories were identified for emotional fatigue and interference with daily life: low and high level of symptoms that were persistent over time. Only one trajectory with a low level of persistent symptoms was identified for cognitive fatigue and social sequelae. Fit parameters for model selection are provided in Supplementary Table S2.
Patients’ characteristics within each cancer-related fatigue trajectory are presented in Table 1. Patients with more comorbidities, advanced pathological tumour stage, higher Clavien–Dindo classification, anxiety, depression, more pain and insomnia symptoms were overrepresented in the high level of cancer-related fatigue trajectory.
Factors associated with cancer-related fatigue trajectories
Older age was associated with having a low level of QLQ-C30 fatigue (OR = 0.96, 95% CI 0.94–0.99), QLQ-FA12 overall (OR 0.96, 95% CI 0.93–0.99), physical (OR = 0.97, 95% CI 0.94–1.00), and emotional (OR = 0.97, 95% CI 0.94–0.99) fatigue trajectory (Table 2). Having more comorbidities was associated with a higher level of QLQ-FA12 overall fatigue (Charlson comorbidity index ≥2 versus 0, those with index, OR = 2.73, 95% CI 1.29–5.78). Pathological tumour Stage III–IV was associated with a higher level of QLQ-C30 fatigue (OR = 2.25, 95% CI 1.28–3.97), QLQ-FA12 overall (OR = 2.33, 95% CI 1.36–4.01) and physical (OR = 1.83, 95% CI 1.08–3.12) fatigue trajectory, compared to Stages 0–I. Patients with more early postoperative complications were more likely to have a high level of emotional fatigue trajectory (Clavien–Dindo classification II–IIIa versus 0–I: OR = 1.78, 95% CI 1.09–2.91) (Table 2).
When further including patient-reported outcome factors in the multivariable models (Table 3), the effects of the Charlson comorbidity index, pathological tumour stage, and Clavien–Dindo classification remained as displayed in Table 2, while the effect of age disappeared. Anxiety was associated with 7 times the odds of having a high level of QLQ-FA12 overall fatigue trajectory (OR = 7.58, 95% CI 2.20–26.17). Depression was associated with about 15-fold increased odds of a high level of QLQ-FA12 overall fatigue trajectory (OR = 15.90, 95% CI 4.44–56.93). Patients with more pain symptoms also showed increased odds of higher-level trajectories for all cancer-related fatigue measurements. Patients with more insomnia symptoms had increased odds of having higher-level trajectories for QLQ-C30 fatigue (OR = 1.01, 95% CI 1.00–1.02) and QLQ-FA12 emotional fatigue (OR = 1.01, 95% CI 1.00–1.02). Preoperative BMI adjusted weight loss grading system and physical activity level 1 year after the surgery did not seem to be associated with cancer-related trajectories (Supplementary Table S3).
Sensitivity analyses
There were 71 (17%) out of the 409 patients who had a single measurement for fatigue. After excluding them, the identified trajectories (Supplementary Fig. S1) and the associations with the potential factors (Supplementary Table S4) remained similar as the main results.
Discussion
Two distinct long-term trajectories of cancer-related fatigue were identified among oesophageal cancer survivors after oesophagectomy, and different longitudinal patterns were presented by specific fatigue dimensions. Clinical factors, including more comorbidities, advanced pathological tumour stage, and postoperative complications, as well as modifiable patient-reported outcome factors one year after surgery, especially anxiety, depression and pain, were found to be associated with the high level of cancer-related fatigue trajectory.
Strengths of this study are the nationwide study design, up to 5-year follow-up, validated and repeated measurements of cancer-related fatigue, and comprehensive and robust data collection including not only sociodemographic and clinical factors but also more subjective, patient-reported variables. Limitations of this study should also be acknowledged. First, the risk of selection biases may arise since some eligible patients were not reachable, declined to consent, or were too weak to join. Attrition during the follow-up is another issue when patients dropped out due to death, cancer recurrence, or unknown reasons. In addition, some of the included patients did not have data for all follow-up time points but were still included in the analysis. Some of the missing were due to the surgical calendar period (e.g., patients who underwent surgery in 2019 have not reached the 5-year follow-up), which is an observed variable and may have limited influence on fatigue measurements during the study period. Growth mixture models enable using all available information and can provide robust estimates given the missing data was at random (missing depends on observed variables). Second, cancer-related fatigue level might also be influenced by cancer recurrence and the accompanied treatments during follow-up, which were not available in this cohort. Such patients may have higher level of fatigue, and could contribute to or cluster in the high-level fatigue trajectory. Some lifestyle and biochemical factors that might influence cancer-related fatigue were also not available, and the multivariable analyses were exploratory in nature without targeted confounder adjustment and complex interaction terms. Thus, unmeasured or residual confounding is inevitable in this observational study. A large number of factors including important patient-reported outcomes were incorporated in the analysis and should relieve the concern to some extent. But some of the involved variables might be intercorrelated and the potential implications were still unknown. Third, a matched background population was used to calculate a proxy baseline fatigue measurement for each oesophageal cancer patient because it is not practical to obtain fatigue measurements at baseline before cancer diagnosis. In addition, without measurements before and during the first year after the surgery, no evolution pattern could be modelled during the treatment and the acute recovery period. Besides, though validated questionnaires were used, the measurement of the fatigue level depended on the subjective individual perceptions of this symptom and inherent variability might exist, which could not be captured by the mean score estimate for each trajectory. Furthermore, the longitudinal change of the subjective fatigue perception, i.e., response shift [42], could further complicate the variability that was difficult to account for. Finally, the individual trajectory of each patient is not fixed and patients were assigned to a trajectory with a posterior probability. Such uncertainty may lead to underestimation of the following associations but was partly accounted for by using weighted regression methods.
To our knowledge, this is one of the first and largest studies regarding longitudinal cancer-related fatigue trajectories among oesophageal cancer patients. Two distinct trajectories were identified for overall and dimensional cancer-related fatigue, while for cognitive fatigue and social sequelae, only one trajectory was found. The numbers of the identified trajectories in this study were less than in previous studies on fatigue among breast cancer [43] and Hodgkin’s lymphoma [38] survivors. This may be partly explained by the limit of the minimum sample size (≥15%) required within each trajectory was larger in the current study than in other studies, which guaranteed sounder and more stable estimates. The fatigue level of the identified trajectories was rather persistent or even deteriorating during the follow-up, without a sign of symptom alleviation, especially in the trajectory with a high level of cancer-related fatigue. Such findings suggest the possibility of using an initial measurement for early identification of the patients within long-term poor cancer-related fatigue trajectory. Personalised cancer survivorship care with timely intervention after early patient identification may prevent them from the long-term fatigue burden.
This study found older patients were associated with a low level of cancer-related fatigue trajectories. However, patients of older age are less prone to be affected by emotional distress after cancer diagnosis [44,45,46], and the association in this study disappeared after adjusting for patient-reported outcome factors, suggesting that the effect of age might be due to confounding or mediation. Proxy fatigue score before cancer diagnosis did not influence long-term cancer-related fatigue in this study, while findings from other studies suggested that fatigue measurements after diagnosis influenced the longitudinal fatigue development [32, 34, 38], which indicated that the timing when measuring the baseline (initial) fatigue level seem to play an important role. Vulnerable patients with more comorbidities [32, 43], advanced tumour stage [47], and postoperative complications [16, 48] are associated with having a higher level of cancer-related fatigue, which was also seen in this study.
Cancer-related fatigue seldom occurs alone and often clusters with other symptoms, including anxiety, depression, pain, and insomnia [31, 34, 43, 49]. This study also found that patients reporting a higher level of these symptoms one year after the surgery were at an increased risk of having a poor long-term cancer-related fatigue trajectory. This calls for the timely monitoring of clustered post-treatment symptoms. Since no Gold-Standard treatment is established for cancer-related fatigue, a comprehensive interventional programme, such as cognitive-behavioural therapy, addressing concurrent symptoms that are easier to modify may help not only relieve the fatigue symptom but also improve the overall cancer survivorship [50]. In addition, given the current healthcare workforce shortage [51, 52], the development and implementation of digital self-management applications are expected to share the workload and are recommended to be incorporated into post-discharge care. The one-time measurement of physical activity did not influence the longitudinal fatigue patterns [33, 43], which underlined the effect of long-term systematic exercise [53].
Understanding the separable long-term development of cancer-related fatigue is the basis for patient stratification and tailored care among cancer survivors. This population-based and longitudinal cohort study revealed distinct 5-year cancer-related fatigue trajectories among oesophageal cancer survivors. More comorbidities, advanced pathological tumour stage, postoperative complications, and self-reported symptoms of anxiety, depression, and pain, seem to influence the fatigue trajectory belonging. Early identification of high-risk patients using the factors found to be associated with a high level of cancer-related fatigue trajectory, and a personalised and comprehensive interventional programme targeting the clustered symptoms are warranted.
Data availability
The datasets generated and analysed in the current study are not publicly available due to ethical restrictions but are available from the corresponding author on reasonable request.
Code availability
All statistical analyses were calculated using standard methods, therefore the code is not provided.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–96.
van Putten M, de Vos-Geelen J, Nieuwenhuijzen GAP, Siersema PD, Lemmens V, Rosman C, et al. Long-term survival improvement in oesophageal cancer in the Netherlands. Eur J Cancer. 2018;94:138–47.
Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol. 2016;31:1141–6.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75.
Haverkamp L, Ruurda JP, van Leeuwen MS, Siersema PD, van Hillegersberg R. Systematic review of the surgical strategies of adenocarcinomas of the gastroesophageal junction. Surg Oncol. 2014;23:222–8.
Rutegard M, Charonis K, Lu Y, Lagergren P, Lagergren J, Rouvelas I. Population-based esophageal cancer survival after resection without neoadjuvant therapy: an update. Surgery. 2012;152:903–10.
Gottlieb-Vedi E, Kauppila JH, Mattsson F, Lindblad M, Nilsson M, Lagergren P, et al. Long-term survival in esophageal cancer after minimally invasive esophagectomy compared to open esophagectomy. Ann Surg. 2021;276:e744–8.
Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. 2020;31:713–23.
Inc. NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Cancer-related fatigue version 2.2022 2022 [cited 2022 0704].
Schandl A, Johar A, Anandavadivelan P, Vikstrom K, Malberg K, Lagergren P. Patient-reported outcomes 1 year after oesophageal cancer surgery. Acta Oncol. 2020;59:613–9.
Djarv T, Lagergren P. Quality of life after esophagectomy for cancer. Expert Rev Gastroenterol Hepatol. 2012;6:115–22.
Schandl A, Lagergren J, Johar A, Lagergren P. Health-related quality of life 10 years after oesophageal cancer surgery. Eur J Cancer. 2016;69:43–50.
Bower JE. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609.
Ebede CC, Jang Y, Escalante CP. Cancer-related fatigue in cancer survivorship. Med Clin North Am. 2017;101:1085–97.
Cheng Z, Johar A, Nilsson M, Lagergren P. Cancer-related fatigue after esophageal cancer surgery: impact of postoperative complications. Ann Surg Oncol. 2022;29:2842–51.
Derogar M, van der Schaaf M, Lagergren P. Reference values for the EORTC QLQ-C30 quality of life questionnaire in a random sample of the Swedish population. Acta Oncol. 2012;51:10–6.
Weis J, Tomaszewski KA, Hammerlid E, Ignacio Arraras J, Conroy T, Lanceley A, et al. International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ-FA12). J Natl Cancer Inst. 2017;109:djw273.
Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol. 2009;20:17–25.
Kuliś D, Bottomley A, Velikova G, Greimel E, Koller M, on behalf of the EORTC Quality of Life Group. EORTC quality of life group translation procedure, Fourth edition. Brussels: European Organisation for Research and Treatment of Cancer; 2017.
Fayers PM, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 scoring manual, Third edition. Brussels: European Organisation for Research and Treatment of Cancer; 2001.
Armitage JN, van der Meulen JH, Royal College of Surgeons Co-morbidity Consensus G. Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg. 2010;97:772–81.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. 2015;33:90–9.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
Klevebro F, Johar A, Lagergren P. Impact of co-morbidities on health-related quality of life 10 years after surgical treatment of oesophageal cancer. BJS Open. 2020;4:601–4.
Sunde B, Klevebro F, Johar A, Johnsen G, Jacobsen AB, Glenjen NI, et al. Health-related quality of life in a randomized trial of neoadjuvant chemotherapy or chemoradiotherapy plus surgery in patients with oesophageal cancer (NeoRes trial). Br J Surg. 2019;106:1452–63.
Schandl AR, Johar A, Mälberg K, Lagergren P. Education level and health-related quality of life after oesophageal cancer surgery: a nationwide cohort study. BMJ Open. 2018;8:e020702.
Kidane B, Ali A, Sulman J, Wong R, Knox JJ, Darling GE. Health-related quality of life measure distinguishes between low and high clinical T stages in esophageal cancer. Ann Transl Med. 2018;6:270.
Bean HR, Diggens J, Ftanou M, Weihs KL, Stanton AL, Wiley JF. Insomnia and fatigue symptom trajectories in breast cancer: a longitudinal cohort study. Behav Sleep Med. 2021;19:814–27.
Poort H, de Rooij BH, Uno H, Weng S, Ezendam NPM, van de Poll-Franse L, et al. Patterns and predictors of cancer-related fatigue in ovarian and endometrial cancers: 1-year longitudinal study. Cancer. 2020;126:3526–33.
Eyl RE, Thong MSY, Carr PR, Jansen L, Koch-Gallenkamp L, Hoffmeister M, et al. Physical activity and long-term fatigue among colorectal cancer survivors—a population-based prospective study. BMC Cancer. 2020;20:438.
Susanne K, Michael F, Thomas S, Peter E, Andreas H. Predictors of fatigue in cancer patients: a longitudinal study. Support Care Cancer. 2019;27:3463–71.
Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38.
Paksarian D, Cui L, Angst J, Ajdacic-Gross V, Rossler W, Merikangas KR. Latent trajectories of common mental health disorder risk across 3 decades of adulthood in a population-based cohort. JAMA Psychiatry. 2016;73:1023–31.
Gueorguieva R, Chekroud AM, Krystal JH. Trajectories of relapse in randomised, placebo-controlled trials of treatment discontinuation in major depressive disorder: an individual patient-level data meta-analysis. Lancet Psychiatry. 2017;4:230–7.
Kreissl S, Mueller H, Goergen H, Mayer A, Brillant C, Behringer K, et al. Cancer-related fatigue in patients with and survivors of Hodgkin’s lymphoma: a longitudinal study of the German Hodgkin Study Group. Lancet Oncol. 2016;17:1453–62.
van der Nest G, Lima Passos V, Candel MJJM, van Breukelen GJP. An overview of mixture modelling for latent evolutions in longitudinal data: modelling approaches, fit statistics and software. Adv Life Course Res. 2020;43:100323.
Giesinger JM, Loth FLC, Aaronson NK, Arraras JI, Caocci G, Efficace F, et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J Clin Epidemiol. 2020;118:1–8.
Friedrich M, Hinz A, Kuhnt S, Schulte T, Rose M, Fischer F. Measuring fatigue in cancer patients: a common metric for six fatigue instruments. Qual Life Res. 2019;28:1615–26.
Vanier A, Oort FJ, McClimans L, Ow N, Gulek BG, Bohnke JR, et al. Response shift in patient-reported outcomes: definition, theory, and a revised model. Qual Life Res. 2021;30:3309–22.
Vaz-Luis I, Di Meglio A, Havas J, El-Mouhebb M, Lapidari P, Presti D, et al. Long-term longitudinal patterns of patient-reported fatigue after breast cancer: a group-based trajectory analysis. J Clin Oncol. 2022;40:2148–62.
Avis NE, Levine B, Naughton MJ, Case DL, Naftalis E, Van Zee KJ. Explaining age-related differences in depression following breast cancer diagnosis and treatment. Breast Cancer Res Treat. 2012;136:581–91.
Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141:343–51.
Hinz A, Herzberg PY, Lordick F, Weis J, Faller H, Brähler E, et al. Age and gender differences in anxiety and depression in cancer patients compared with the general population. Eur J Cancer Care. 2019;28:e13129.
Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen C, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27:965–74.
Kauppila JH, Johar A, Lagergren P. Postoperative complications and health-related quality of life 10 years after esophageal cancer surgery. Ann Surg. 2020;271:311–6.
Bower JE, Ganz PA, Irwin MR, Cole SW, Garet D, Petersen L, et al. Do all patients with cancer experience fatigue? A longitudinal study of fatigue trajectories in women with breast cancer. Cancer. 2021;127:1334–44.
Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961–8.
Mayer DK, Alfano CM. Personalized risk-stratified cancer follow-up care: its potential for healthier survivors, happier clinicians, and lower costs. J Natl Cancer Inst. 2019;111:442–8.
Takvorian SU, Balogh E, Nass S, Valentin VL, Hoffman-Hogg L, Oyer RA, et al. Developing and sustaining an effective and resilient oncology careforce: opportunities for action. J Natl Cancer Inst. 2020;112:663–70.
van Roekel EH, Duchâteau J, Bours MJL, van Delden L, Breedveld-Peters JJL, Koole JL, et al. Longitudinal associations of light-intensity physical activity with quality of life, functioning and fatigue after colorectal cancer. Qual Life Res. 2020;29:2987–98.
Acknowledgements
The authors wish to thank the participating patients and their families as well as the hospital departments and cancer centres for their contributions to the study. The authors also wish to thank the patient partnership group for their excellent suggestions.
Funding
This study is funded by the Swedish Cancer Society, the Swedish Research Council, and the Cancer Research Funds of Radiumhemmet. ZC is supported by the China Scholarship Council. Pernilla Lagergren is supported by NIHR Imperial Biomedical Research Centre (BRC) for her Imperial College London affiliation. Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
ZC: conceptualisation, formal analysis, methodology, writing—original draft, writing—review and editing. AJ: data curation, methodology, writing—review and editing. MN: writing—review and editing. AS: resources, writing—review and editing. PL: conceptualisation, funding acquisition, project administration, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki. The project was approved by the Regional Ethical Review Board in Stockholm (2013/844-31/1). Informed consent forms were obtained from all participants.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, Z., Johar, A., Nilsson, M. et al. Cancer-related fatigue trajectories up to 5 years after curative treatment for oesophageal cancer. Br J Cancer 130, 628–637 (2024). https://doi.org/10.1038/s41416-023-02551-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02551-0