Abstract
Background
The expression of Egl-9 family hypoxia-inducible factor 3 (EGLN3) is notably decreased in various malignancies, including gastric cancer (GC). While the predominant focus has been on the hydroxylase activity of EGLN3 for its antitumour effects, recent findings have suggested nonenzymatic roles for EGLN3.
Methods
This study assessed the clinical significance of EGLN3 expression in GC and explored the connection between EGLN3 DNA promoter methylation and transcriptional silencing. To investigate the effect of EGLN3 on GC cells, a gain-of-function strategy was adopted. RNA sequencing was conducted to identify the key effector molecules and signalling pathways associated with EGLN3.
Results
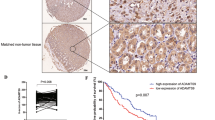
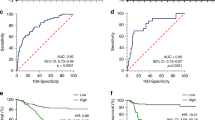
EGLN3 expression was significantly reduced in GC tissues, correlating with poorer patient prognosis. EGLN3 hypermethylation disrupts transcriptional equilibrium, contributing to deeper tumour invasion and lymph node metastasis, thus exacerbating GC progression. Conversely, restoration of EGLN3 expression in GC cells substantially inhibited cell proliferation and metastasis. EGLN3 was also found to impede the malignant progression of GC cells by downregulating Jumonji C domain-containing protein 8-mediated activation of the NF-κB pathway, independent of its hydroxylase activity.
Conclusions
EGLN3 has the potential to hinder the spread of GC cells through a nonenzymatic mechanism, thereby shedding light on the complex nature of GC progression.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
GSE200646 (GEO database).
References
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–48.
Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18:534–42.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Pescador N, Cuevas Y, Naranjo S, Alcaide M, Villar D, Landázuri MO, et al. Identification of a functional hypoxia-responsive element that regulates the expression of the egl nine homologue 3 (egln3/phd3) gene. Biochem J. 2005;390:189–97.
Jaakkola PM, Rantanen K. The regulation, localization, and functions of oxygen-sensing prolyl hydroxylase PHD3. Biol Chem. 2013;394:449–57.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8.
Place TL, Domann FE. Prolyl-hydroxylase 3: evolving roles for an ancient signalling protein. Hypoxia (Auckl). 2013;2013:13–17.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54.
Flashman E, Bagg EA, Chowdhury R, Mecinović J, Loenarz C, McDonough MA, et al. Kinetic rationale for selectivity toward N- and C-terminal oxygen-dependent degradation domain substrates mediated by a loop region of hypoxia-inducible factor prolyl hydroxylases. J Biol Chem. 2008;283:3808–15.
Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA. 2001;98:9630–5.
Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–65.
Dopeso H, Jiao HK, Cuesta AM, Henze AT, Jurida L, Kracht M, et al. PHD3 controls lung cancer metastasis and resistance to EGFR inhibitors through TGFα. Cancer Res. 2018;78:1805–19.
Chiba N, Sunamura M, Nakagawa M, Koganezawa I, Yokozuka K, Kobayashi T, et al. Overexpression of hydroxyproline via EGLN/HIF1A is associated with distant metastasis in pancreatic cancer. Am J Cancer Res. 2020;10:2570–81.
German NJ, Yoon H, Yusuf RZ, Murphy JP, Finley LW, Laurent G, et al. PHD3 loss in cancer enables metabolic reliance on fatty acid oxidation via deactivation of ACC2. Mol cell. 2016;63:1006–20.
Rodriguez J, Herrero A, Li S, Rauch N, Quintanilla A, Wynne K, et al. PHD3 regulates p53 protein stability by hydroxylating proline 359. Cell Rep. 2018;24:1316–29.
Xue J, Li X, Jiao S, Wei Y, Wu G, Fang J, et al. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKbeta independent of hydroxylase activity. Gastroenterology. 2010;138:606–15.
Klutstein M, Nejman D, Greenfield R, Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016;76:3446–50.
Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–92.
Ma G, Jing C, Li L, Huang F, Ding F, Wang B, et al. MicroRNA-92b represses invasion-metastasis cascade of esophageal squamous cell carcinoma. Oncotarget. 2016;7:20209–22.
Grady WM, Yu M, Markowitz SD. Epigenetic alterations in the gastrointestinal tract: current and emerging use for biomarkers of cancer. Gastroenterology. 2021;160:690–709.
Tanaka T, Li TS, Urata Y, Goto S, Ono Y, Kawakatsu M, et al. Increased expression of PHD3 represses the HIF-1 signaling pathway and contributes to poor neovascularization in pancreatic ductal adenocarcinoma. J Gastroenterol. 2015;50:975–83.
Tang LR, Wu JX, Cai SL, Huang YX, Zhang XQ, Fu WK, et al. Prolyl hydroxylase domain 3 influences the radiotherapy efficacy of pancreatic cancer cells by targeting hypoxia-inducible factor-1α. OncoTargets Ther. 2018;11:8507–15.
Shi M, Dai WQ, Jia RR, Zhang QH, Wei J, Wang YG, et al. APC(CDC20)-mediated degradation of PHD3 stabilizes HIF-1a and promotes tumorigenesis in hepatocellular carcinoma. Cancer Lett. 2021;496:144–55.
Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40.
Zhang B, Zhang Y, Jiang X, Su H, Wang Q, Wudu M, et al. JMJD8 promotes malignant progression of lung cancer by maintaining EGFR stability and EGFR/PI3K/AKT pathway activation. J Cancer. 2021;12:976–87.
Yi J, Wang L, Du J, Wang M, Shen H, Liu Z, et al. ER-localized JmjC domain-containing protein JMJD8 targets STING to promote immune evasion and tumour growth in breast cancer. Dev Cell. 2023;58:760–78.e6.
Yeo KS, Tan MC, Wong WY, Loh SW, Lam YL, Tan CL, et al. JMJD8 is a positive regulator of TNF-induced NF-κB signalling. Sci Rep. 2016;6:34125.
Rawluszko AA, Bujnicka KE, Horbacka K, Krokowicz P, Jagodziński PP. Expression and DNA methylation levels of prolyl hydroxylases PHD1, PHD2, PHD3 and asparaginyl hydroxylase FIH in colorectal cancer. BMC cancer. 2013;13:526.
Place TL, Fitzgerald MP, Venkataraman S, Vorrink SU, Case AJ, Teoh ML, et al. Aberrant promoter CpG methylation is a mechanism for impaired PHD3 expression in a diverse set of malignant cells. PloS one. 2011;6:e14617.
Xia X, Wang S, Ni B, Xing S, Cao H, Zhang Z, et al. Hypoxic gastric cancer-derived exosomes promote progression and metastasis via MiR-301a-3p/PHD3/HIF-1α positive feedback loop. Oncogene. 2020;39:6231–44.
Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39.
Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–82.
Peng G, Wang Y, Ge P, Bailey C, Zhang P, Zhang D, et al. The HIF1α-PDGFD-PDGFRα axis controls glioblastoma growth at normoxia/mild-hypoxia and confers sensitivity to targeted therapy by echinomycin. J Exp Clin Cancer Res. 2021;40:278.
Park JH, Kim TY, Jong HS, Kim TY, Chun YS, Park JW, et al. Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1alpha in gastric cancer cells. Clin Cancer Res. 2003;9:433–40.
Lee YH, Bae HC, Noh KH, Song KH, Ye SK, Mao CP, et al. Gain of HIF-1α under normoxia in cancer mediates immune adaptation through the AKT/ERK and VEGFA axes. Clin Cancer Res. 2015;21:1438–46.
Yeo KS, Tan MC, Lim YY, Ea CK. JMJD8 is a novel endoplasmic reticulum protein with a JmjC domain. Sci Rep. 2017;7:15407.
Accari SL, Fisher PR. Emerging roles of JmjC domain-containing proteins. Int Rev Cell Mol Biol. 2015;319:165–220.
Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–6.
Oh S, Shin S, Janknecht R. The small members of the JMJD protein family: enzymatic jewels or jinxes? Biochim Biophys Acta Rev Cancer. 2019;1871:406–18.
Boeckel JN, Derlet A, Glaser SF, Luczak A, Lucas T, Heumüller AW, et al. JMJD8 regulates angiogenic sprouting and cellular metabolism by interacting with pyruvate kinase M2 in endothelial cells. Arterioscler Thromb Vasc Biol. 2016;36:1425–33.
Wang L, Jiang F, Ma F, Zhang B. MiR-873-5p suppresses cell proliferation and epithelial-mesenchymal transition via directly targeting Jumonji domain-containing protein 8 through the NF-κB pathway in colorectal cancer. J cell Commun Signal. 2019;13:549–60.
Su Y, Wang X, Guo Z, Wang J. Aberrant JmjC domain-containing protein 8 (JMJD8) expression promotes activation of AKT and tumour epithelial-mesenchymal transition. Oncogene. 2020;39:6451–67.
Lee BL, Lee HS, Jung J, Cho SJ, Chung HY, Kim WH, et al. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin Cancer Res. 2005;11:2518–25.
Soutto M, Bhat N, Khalafi S, Zhu S, Poveda J, Garcia-Buitrago M, et al. NF-kB-dependent activation of STAT3 by H. pylori is suppressed by TFF1. Cancer Cell Int. 2021;21:444.
Shen Y, Xue C, Li X, Ba L, Gu J, Sun Z, et al. Effects of gastric cancer cell-derived exosomes on the immune regulation of mesenchymal stem cells by the NF-kB signalling pathway. Stem Cells Dev. 2019;28:464–76.
Xia L, Tan S, Zhou Y, Lin J, Wang H, Oyang L, et al. Role of the NFκB-signaling pathway in cancer. OncoTargets Ther. 2018;11:2063–73.
Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR, et al. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune. Dis Genes. 2021;8:287–97.
Acknowledgements
Thanks for all help.
Funding
This work was jointly funded by the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A), the Distinguished professor of Tianjin (JTZB [2019] No.120), the Programs of National Natural Science Foundation of China (No. 81572372), the National Natural Science Foundation of China (81974373).
Author information
Authors and Affiliations
Contributions
FC and JD were major contributors to the design of this study and drafted the manuscript. GM, YN, CD and JD supported the research techniques. NZ, XY, PW, and MZ were responsible for tissue collection. RZ, HL and JD were responsible for clinical databases. JD designed this study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The use of specimens from patients were approved by the Institutional Research Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (no. bc2020091, date: 27 July 2020) (Tianjin, China). Informed consent was obtained for the collection of all samples in accordance with the Declaration of Helsinki. All experimental animal procedures were carried out with the approval of the Institutional Animal Care and Research Advisory Committee of Tianjin Medical University (Tianjin, China) under grant no. AE-2021073 (date: 11 October 2021).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, F., Yang, X., Ma, G. et al. EGLN3 attenuates gastric cancer cell malignant characteristics by inhibiting JMJD8/NF-κB signalling activation independent of hydroxylase activity. Br J Cancer 130, 597–612 (2024). https://doi.org/10.1038/s41416-023-02546-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02546-x