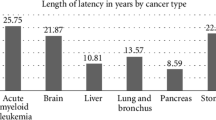
Abstract
Background
In estimating radiation-associated cancer risks a fixed period for the minimum latency is often assumed. Two empirical latency functions have been used to model latency, continuously increasing from 0. A stochastic biologically-based approach yields a still more plausible way of describing latency and can be directly estimated from clinical data.
Methods
We derived the parameters for a stochastic biologically-based model from tumour growth data for various cancers, and least-squares fitted the two types of empirical latency function to the stochastic model-predicted cumulative probability.
Results
There is wide variation in growth rates among tumours, particularly slow for prostate and thyroid cancer and particularly fast for leukaemia. The slow growth rate for prostate and thyroid tumours implies that the number of tumour cells required for clinical detection cannot greatly exceed 106. For all tumours, both empirical latency functions closely approximated the predicted biological model cumulative probability.
Conclusions
Our results, illustrating use of a stochastic biologically-based model using clinical data not tied to any particular carcinogen, have implications for estimating latency associated with any mutagen. They apply to tumour growth in general, and may be useful for example, in planning screenings for cancer using imaging techniques.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data used are given in Table 1, also in Appendix B, and are derived from the peer-reviewed literature.
Code availability
The various calculations used are given in an Excel spreadsheet, provided in Appendix B.
References
United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2006 Report. Annex A. Epidemiological Studies of Radiation and Cancer. E.08.IX.6. 13-322. New York: United Nations; 2008.
International Commission on Radiological Protection (ICRP). The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37:1–332.
International Commission on Radiological Protection. Radiation detriment calculation methodology. ICRP publication 152. Ann ICRP. 2022;51:9–103.
Kocher DC, Apostoaei AI, Henshaw RW, Hoffman FO, Schubauer-Berigan MK, Stancescu DO, et al. Interactive RadioEpidemiological Program (IREP): a web-based tool for estimating probability of causation/assigned share of radiogenic cancers. Health Phys. 2008;95:119–47.
Land C, Gilbert E, Smith JM, Hoffman FO, Apostoaei I, Thomas B, et al. Report of the NCI-CDC Working Group to Revise the 1985 NIH Radioepidemiological Tables 1-118. U.S. Department of Health and Human Services; 2003.
Berrington de Gonzalez A, Apostoaei AI, Veiga LHS, Rajaraman P, Thomas BA, Hoffman FO, et al. RadRAT: a radiation risk assessment tool for lifetime cancer risk projection. J Radiol Prot. 2012;32:205–22.
Ulanowski A, Shemiakina E, Güthlin D, Becker J, Preston D, Apostoaei AI, et al. ProZES: the methodology and software tool for assessment of assigned share of radiation in probability of cancer occurrence. Radiat Environ Biophys. 2020;59:601–29.
DeVita VT Jr, Young RC, Canellos GP. Combination versus single agent chemotherapy: a review of the basis for selection of drug treatment of cancer. Cancer. 1975;35:98–110.
Del Monte U. Does the cell number 109 still really fit one gram of tumor tissue? Cell Cycle. 2009;8:505–6.
Little MP, Kleinerman RA, Stiller CA, Li G, Kroll ME, Murphy MFG. Analysis of retinoblastoma age incidence data using a fully stochastic cancer model. Int J Cancer. 2012;130:631–40.
Gauss CF, Besprechung des Buchs von LA. Seeber: Untersuchungen über die Eigenschaften der positiven ternären quadratischen Formen usw [Discussion of L. A. Seeber’s book: Studies on the characteristics of positive ternary quadratic forms etc]. Göttingsche Gelehrte Anz. 1831;108:1065–77.
Grantab R, Sivananthan S, Tannock IF. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Res. 2006;66:1033–9.
Grantab RH, Tannock IF. Penetration of anticancer drugs through tumour tissue as a function of cellular packing density and interstitial fluid pressure and its modification by bortezomib. BMC Cancer. 2012;12:214.
Grosser S, Lippoldt J, Oswald L, Merkel M, Sussman DM, Renner F, et al. Cell and nucleus shape as an indicator of tissue fluidity in carcinoma. Phys Rev X. 2021;11:011033.
Excel 2021 version 2102 build 13801.21050 (Office 365 Apps for enterprise). Seattle, WA: Microsoft; 2021.
Heuser L, Spratt JS, Polk HC Jr. Growth rates of primary breast cancers. Cancer. 1979;43:1888–94.
Egawa S, Matsumoto K, Iwamura M, Uchida T, Kuwao S, Koshiba K. Impact of life expectancy and tumor doubling time on the clinical significance of prostate cancer in Japan. Jpn J Clin Oncol. 1997;27:394–400.
Schmid H-P, McNeal JE, Stamey TA. Observations on the doubling time of prostate cancer. The use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer. 1993;71:2031–40.
Kaigorodova EV, Kozik AV, Zavaruev IS, Grishchenko MY. Hybrid/atypical forms of circulating tumor cells: current state of the art. Biochemistry (Moscow). 2022;87:380–90.
Gompertz B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of Life Contingencies. In a Letter to Francis Baily, Esq. Proc R Soc Lond. 1825;115:513–83.
Winsor CP. The Gompertz curve as a growth curve. Proc Natl Acad Sci USA. 1932;18:1–8.
Laird AK. Dynamics of tumor growth. Br J Cancer. 1964;18:490–502.
Laird AK. Dynamics of tumour growth: comparison of growth rates and extrapolation of growth curve to one cell. Br J Cancer. 1965;19:278–91.
Luebeck EG, Curtius K, Jeon J, Hazelton WD. Impact of tumor progression on cancer incidence curves. Cancer Res. 2013;73:1086–96.
Fakir H, Hofmann W, Sachs RK. Modeling progression in radiation-induced lung adenocarcinomas. Radiat Environ Biophys. 2010;49:169–76.
National Council on Radiation Protection and Measurements (NCRP). Report No. 186. Approaches for integrating information from radiation biology and epidemiology to enhance low-dose health risk assessment. i-xi+1-296. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 2020.
Little MP. Are two mutations sufficient to cause cancer? Some generalizations of the two-mutation model of carcinogenesis of Moolgavkar, Venzon, and Knudson, and of the multistage model of Armitage and Doll. Biometrics. 1995;51:1278–91.
Rühm W, Eidemüller M, Kaiser JC. Biologically-based mechanistic models of radiation-related carcinogenesis applied to epidemiological data. Int J Radiat Biol. 2017;93:1093–117.
Little MP, Wright EG. A stochastic carcinogenesis model incorporating genomic instability fitted to colon cancer data. Math Biosci. 2003;183:111–34.
United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2020/2021 Report. Volume III. Annex C. Biological mechanisms relevant for the inference of cancer risks from low-dose and low-dose-rate radiation. E.22.IX.3 1-238. New York: United Nations; 2021.
Little MP, Vineis P, Li G. A stochastic carcinogenesis model incorporating multiple types of genomic instability fitted to colon cancer data. J Theor Biol. 2008;254:229–38.
Little MP. Cancer models, genomic instability and somatic cellular Darwinian evolution. Biol Direct. 2010;5:19.
Little MP, Wakeford R, Borrego D, French B, Zablotska LB, Adams MJ, et al. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: a pooled analysis of nine historical cohort studies. Lancet Haematol. 2018;5:e346–58.
Fuzik M, Prysyazhnyuk A, Shibata Y, Romanenko A, Fedorenko Z, Gulak L, et al. Thyroid cancer incidence in Ukraine: trends with reference to the Chernobyl accident. Radiat Environ Biophys. 2011;50:47–55.
Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21.
Little MP, Kukush AG, Masiuk SV, Shklyar S, Carroll RJ, Lubin JH, et al. Impact of uncertainties in exposure assessment on estimates of thyroid cancer risk among Ukrainian children and adolescents exposed from the Chernobyl accident. PLoS ONE. 2014;9:e85723.
Little MP, Kwon D, Zablotska LB, Brenner AV, Cahoon EK, Rozhko AV, et al. Impact of uncertainties in exposure assessment on thyroid cancer risk among persons in Belarus exposed as children or adolescents due to the Chernobyl accident. PLoS ONE. 2015;10:e0139826.
Oh H-S, Kwon H, Song E, Jeon MJ, Kim TY, Lee JH, et al. Tumor volume doubling time in active surveillance of papillary thyroid carcinoma. Thyroid. 2019;29:642–9.
Miyauchi A, Kudo T, Ito Y, Oda H, Yamamoto M, Sasai H, et al. Natural history of papillary thyroid microcarcinoma: kinetic analyses on tumor volume during active surveillance and before presentation. Surgery. 2019;165:25–30.
Kihara M, Miyauchi A, Masuoka H, Higashiyama T, Ito Y, Miya A. Kinetic analysis of the growth rate of sporadic and hereditary medullary thyroid carcinoma: comparing the postoperative calcitonin-doubling rate with the hypothetical preoperative tumor volume-doubling rate. Thyroid Res. 2020;13:13.
Eidemüller M, Becker J, Kaiser JC, Ulanowski A, Apostoaei AI, Hoffman FO. Concepts of association between cancer and ionising radiation: accounting for specific biological mechanisms. Radiat Environ Biophys. 2023;62:1–15.
D’Amico AV, Hanks GE. Linear regressive analysis using prostate-specific antigen doubling time for predicting tumor biology and clinical outcome in prostate cancer. Cancer. 1993;72:2638–43.
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–16.
Stamey TA, Kabalin JN. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. I. Untreated patients. J Urol. 1989;141:1070–5.
Bolin S, Nilsson E, Sjödahl R. Carcinoma of the colon and rectum-growth rate. Ann Surg. 1983;198:151–8.
Burke JR, Brown P, Quyn A, Lambie H, Tolan D, Sagar P. Tumour growth rate of carcinoma of the colon and rectum: retrospective cohort study. BJS Open. 2020;4:1200–7.
Knudsen AB, Rutter CM, Peterse EFP, Lietz AP, Seguin CL, Meester RGS, et al. Colorectal cancer screening: an updated decision analysis for the U.S. Preventive Services Task Force. i-ix+1-275. Rockville, MD, USA: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services; 2021.
Nguyen DH, Oketch-Rabah HA, Illa-Bochaca I, Geyer FC, Reis-Filho JS, Mao J-H, et al. Radiation acts on the microenvironment to affect breast carcinogenesis by distinct mechanisms that decrease cancer latency and affect tumor type. Cancer Cell. 2011;19:640–51.
International Commission on Radiological Protection (ICRP). Stem cell biology with respect to carcinogenesis aspects of radiological protection. ICRP Publication 131. Ann ICRP. 2015;44:1–357.
Richardson DB, Ashmore JP. Investigating time patterns of variation in radiation cancer associations. Occup Environ Med. 2005;62:551–8.
Daniels RD, Bertke SJ, Richardson DB, Cardis E, Gillies M, O’Hagan JA, et al. Examining temporal effects on cancer risk in the international nuclear workers’ study. Int J Cancer. 2017;140:1260–9.
Hauptmann M, Wellmann J, Lubin JH, Rosenberg PS, Kreienbrock L. Analysis of exposure-time-response relationships using a spline weight function. Biometrics. 2000;56:1105–8.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Raabe OG. Concerning ionizing radiation-induced cancer from internally deposited radionuclides. Int J Radiat Biol. 2015;91:810–9.
Shuryak I, Hahnfeldt P, Hlatky L, Sachs RK, Brenner DJ. A new view of radiation-induced cancer: integrating short- and long-term processes. Part I: approach. Radiat Environ Biophys 2009;48:263–74.
Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81.
Bucci A, Shore-Freedman E, Gierlowski T, Mihailescu D, Ron E, Schneider AB. Behavior of small thyroid cancers found by screening radiation-exposed individuals. J Clin Endocrinol Metab. 2001;86:3711–6.
Rennau H. Personal communication to Dr Markus Eidemüller. 2023.
Mabuchi K, Preston DL, Brenner AV, Sugiyama H, Utada M, Sakata R, et al. Risk of prostate cancer incidence among atomic bomb survivors: 1958-2009. Radiat Res. 2021;195:66–76.
Liu S, Edgerton SM, Moore DH II, Thor AD. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res. 2001;7:1716–23.
Frei E, Freireich EJ. Progress and perspectives in the chemotherapy of acute leukemia. In: Goldin A, Hawking F, Schnitzer RJ, editors. Advances in chemotherapy. New York: Academic Press; 1965. p. 269–98. https://doi.org/10.1016/B978-1-4831-9930-6.50011-3.
Gregory WM, Richards MA, Slevin ML, Souhami RL. A mathematical model relating response durations to amount of subclinical resistant disease. Cancer Res. 1991;51:1210–6.
Geddes DM. The natural history of lung cancer: a review based on rates of tumour growth. Br J Dis Chest. 1979;73:1–17.
Obayashi K, Shimizu K, Nakazawa S, Nagashima T, Yajima T, Kosaka T, et al. The impact of histology and ground-glass opacity component on volume doubling time in primary lung cancer. J Thorac Dis. 2018;10:5428–34.
Ryu EB, Chang JM, Seo M, Kim SA, Lim JH, Moon WK. Tumour volume doubling time of molecular breast cancer subtypes assessed by serial breast ultrasound. Eur Radiol. 2014;24:2227–35.
Spratt JS, Greenberg RA, Heuser LS. Geometry, growth rates, and duration of cancer and carcinoma in situ of the breast before detection by screening. Cancer Res. 1986;46:970–4.
Kusama S, Spratt JS Jr., Donegan WL, Watson FR, Cunningham C. The gross rates of growth of human mammary carcinoma. Cancer. 1972;30:594–9.
Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10:R41.
Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut. 2021;70:401–7.
Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. 2007;84:5–10.
Waaijer A, Terhaard CHJ, Dehnad H, Hordijk G-J, van Leeuwen MS, Raaymakers CPJ, et al. Waiting times for radiotherapy: consequences of volume increase for the TCP in oropharyngeal carcinoma. Radiother Oncol. 2003;66:271–6.
Acknowledgements
The work of MPL was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The work of MPL, ME, and AIA was done in conjunction with work done for ICRP Task Group TG122. ME would like to thank Dr Hannes Rennau (University of Rostock) for discussions on tumour sizes and development. The authors thank the three referees for their detailed and helpful remarks.
Author information
Authors and Affiliations
Contributions
MPL conceived and designed the study, performed the analysis and assembled the first draft. MPL, AIA, ME and JCK performed literature searches and assembled the analytic database. All authors contributed equally to the writing and editing of subsequent drafts. All authors approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Little, M.P., Eidemüller, M., Kaiser, J.C. et al. Minimum latency effects for cancer associated with exposures to radiation or other carcinogens. Br J Cancer 130, 819–829 (2024). https://doi.org/10.1038/s41416-023-02544-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02544-z