Abstract
Background
Platinum-sensitivity is a phenotypic biomarker of Poly (ADP-ribose) polymerase inhibitors (PARPi) sensitivity in histotypes where PARPi are approved. Approximately one-third of non-small cell lung cancers (NSCLC) are platinum-sensitive. The double-blind, randomized phase II PIPSeN (NCT02679963) study evaluated olaparib, a PARPi, as maintenance therapy for patients with platinum-sensitive advanced NSCLC.
Methods
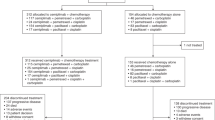
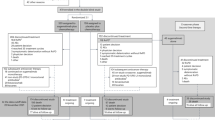
Chemonaïve patients with ECOG performance status of 0–1, platinum-sensitive, EGFR- and ALK-wild-type, stage IIIB-IV NSCLC were randomized (R) to receive either olaparib (O) maintenance or a placebo (P). The primary objective was progression-free survival (PFS) from R. Secondary objectives included overall survival (OS) and safety. With an anticipated hazard ratio of 0.65, 144 patients were required to be randomized, and approximately 500 patients enrolled.
Results
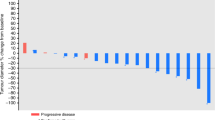
The trial was prematurely terminated because anti-PD(L)1 therapy was approved during the trial recruitment. A total of 182 patients were enrolled, with 60 patients randomized: 33 and 27 in the O and P arms, respectively. Patient and tumor characteristics were well-balanced between arms, except for alcohol intake (33% vs 11% in the O and P arms, respectively, p = 0.043). The median PFS was 2.9 and 2.0 months in the O and P arms, respectively (logrank p = 0.99). The median OS was 9.4 and 9.5 months in the O and P arms, respectively (p = 0.28). Grade ≥3 toxicities occurred in 15 and 8 patients in O and P arms, with no new safety concerns.
Conclusion
PIPSeN was terminated early after enrollment of only 50% of the pre-planned population, thus being statistically underpowered. Olaparib maintenance did neither improve median PFS nor OS in this patient population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Original data could be made available upon request for research purposes on a case-by-case basis, in a pseudonymized way and under current GDPR policies, so that patient protection is fully ensured.
References
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl J Med. 2002;346:92–8.
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–55.
Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40.
Blais N, Kassouf E. Maintenance therapies for non-small cell lung cancer. Front Oncol. 2014;4:213.
Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–8.
Mateo J, Lord CJ, Serra V, Tutt A, Balmana J, Castroviejo-Bermejo M, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30:1437–47.
Govindan R, Lind M, Insa A, Khan SA, Uskov D, Tafreshi A, et al. Veliparib plus carboplatin and paclitaxel versus investigator’s choice of standard chemotherapy in patients with advanced non-squamous non-small cell lung cancer. Clin Lung Cancer. 2022;23:214–25.
Mirza MR, Coleman RL, Gonzalez-Martin A, Moore KN, Colombo N, Ray-Coquard I, et al. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;31:1148–59.
Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, et al. Olaparib plus Bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–28.
Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402.
Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance Olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317–27.
Postel-Vinay S, Bajrami I, Friboulet L, Elliott R, Fontebasso Y, Dorvault N, et al. A high-throughput screen identifies PARP1/2 inhibitors as a potential therapy for ERCC1-deficient non-small cell lung cancer. Oncogene. 2013;32:5377–87.
Postel-Vinay S, Vanhecke E, Olaussen KA, Lord CJ, Ashworth A, Soria JC. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat Rev Clin Oncol. 2012;9:144–55.
Sung M, Giannakakou P. BRCA1 regulates microtubule dynamics and taxane-induced apoptotic cell signaling. Oncogene. 2014;33:1418–28.
Boukovinas I, Papadaki C, Mendez P, Taron M, Mavroudis D, Koutsopoulos A, et al. Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PloS one. 2008;3:e3695.
Edelman MJ, Le Chevalier T, Soria JC. Maintenance therapy and advanced non-small-cell lung cancer: a skeptic’s view. J Thorac Oncol. 2012;7:1331–6.
Azzoli CG, Temin S, Aliff T, Baker S Jr., Brahmer J, Johnson DH, et al. 2011 Focused update of 2009 American Society of clinical oncology clinical practice guideline update on chemotherapy for Stage IV non-small-cell lung cancer. J Clin Oncol. 2011;29:3825–31.
Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, et al. Non-small cell lung cancer. J Natl Compr Cancer Netw. 2010;8:740–801.
Edelman MJ, Harb WA, Pal SE, Boccia RV, Kraut MJ, Bonomi P, et al. Multicenter trial of EC145 in advanced, folate-receptor positive adenocarcinoma of the lung. J Thorac Oncol. 2012;7:1618–21.
Ramalingam SS, Novello S, Guclu SZ, Bentsion D, Zvirbule Z, Szilasi M, et al. Veliparib in combination with platinum-based chemotherapy for first-line treatment of advanced squamous cell lung cancer: a randomized, multicenter Phase III study. J Clin Oncol. 2021;39:3633–44.
Reck M, Blais N, Juhasz E, Gorbunova V, Jones CM, Urban L, et al. Smoking history predicts sensitivity to PARP inhibitor veliparib in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:1098–108.
Fennell DA, Porter C, Lester J, Danson S, Blackhall F, Nicolson M, et al. Olaparib maintenance versus placebo monotherapy in patients with advanced non-small cell lung cancer (PIN): a multicentre, randomised, controlled, phase 2 trial. EClinicalMed. 2022;52:101595.
Owonikoko TK, Redman MW, Byers LA, Hirsch FR, Mack PC, Schwartz LH, et al. Phase 2 study of Talazoparib in patients with homologous recombination repair-deficient squamous cell lung cancer: lung-MAP substudy S1400G. Clin Lung Cancer. 2021;22:187–94.e1.
Chabanon RM, Rouanne M, Lord CJ, Soria JC, Pasero P, Postel-Vinay S. Targeting the DNA damage response in immuno-oncology: developments and opportunities. Nat Rev Cancer. 2021;21:701–17.
Chabanon RM, Muirhead G, Krastev DB, Adam J, Morel D, Garrido M, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Investig. 2019;129:1211–28.
Sonnenblick A, de Azambuja E, Azim HA Jr., Piccart M. An update on PARP inhibitors–moving to the adjuvant setting. Nat Rev Clin Oncol. 2015;12:27–41.
Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137–54.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61.
Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance Olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505.
de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102.
Sokol ES, Pavlick D, Khiabanian H, Frampton GM, Ross JS, Gregg JP, et al. Pan-cancer analysis of BRCA1 and BRCA2 genomic alterations and their association with genomic instability as measured by genome-wide loss of heterozygosity. JCO Precis Oncol. 2020;4:442–65.
Heeke AL, Pishvaian MJ, Lynce F, Xiu J, Brody JR, Chen WJ, et al. Prevalence of homologous recombination-related gene mutations across multiple cancer types. JCO Precis Oncol. 2018;2.
Westphalen CB, Fine AD, Andre F, Ganesan S, Heinemann V, Rouleau E, et al. Pan-cancer analysis of homologous recombination repair-associated gene alterations and genome-wide loss-of-heterozygosity score. Clin Cancer Res. 2022;28:1412–21.
Remon J, Besse B, Leary A, Bieche I, Job B, Lacroix L, et al. Somatic and Germline BRCA 1 and 2 mutations in advanced NSCLC from the SAFIR02-lung trial. JTO Clin Res Rep. 2020;1:100068.
Lok BH, Gardner EE, Schneeberger VE, Ni A, Desmeules P, Rekhtman N, et al. PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin Cancer Res. 2017;23:523–35.
Winkler C, Armenia J, Jones GN, Tobalina L, Sale MJ, Petreus T, et al. SLFN11 informs on standard of care and novel treatments in a wide range of cancer models. Br J cancer. 2021;124:951–62.
Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368:1101–10.
Ma D, Baruch D, Shu Y, Yuan K, Sun Z, Ma K, et al. Using protein microarray technology to screen anti-ERCC1 monoclonal antibodies for specificity and applications in pathology. BMC Biotechnol. 2012;12:88.
Rafiei S, Fitzpatrick K, Liu D, Cai MY, Elmarakeby HA, Park J, et al. ATM loss confers greater sensitivity to ATR inhibition than PARP inhibition in prostate cancer. Cancer Res. 2020;80:2094–100.
Besse B, Awad MM, Forde PM, Thomas M, Goss G, Aronson B, et al. OA15.05 HUDSON: an open-label, multi-drug, biomarker-directed Phase 2 study in NSCLC patients who progressed on Anti-PD-(L)1 therapy. J Thorac Oncol. 2022;17:S41–S2.
Acknowledgements
Figure 1 has been created by Mihaela Aldea with Biorender.com. English editing was performed with ChatGPT v4.
Author information
Authors and Affiliations
Contributions
Study design, SPV, JCS and RR; Methodology & statistics, SPV, JP, MT, JCS and RR; Investigation, patient screening and enrollment: SPV, JC, AG, MD, DP, RDLP, MASG, SV, JP, ANO, TM, CC, ALM, MP, JCS, BB, BM, RR; Writing – Original Draft: MA and SPV; Writing – Review & Editing; all co-authors; Funding Acquisition, SPV and JCS.
Corresponding author
Ethics declarations
Competing interests
SPV and AG: Principal/sub-Investigator of Clinical Trials for Abbvie, Adaptimmune, Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Arno Therapeutics, Astex Pharmaceuticals, Astra Zeneca Ab, Aveo, Basilea Pharmaceutica International Ltd, Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Ca, Celgene Corporation, Chugai Pharmaceutical Co, Cullinan-Apollo, Daiichi Sankyo, Debiopharm, Eisai, Eisai Limited, Eli Lilly, Exelixis, Forma Tharapeutics, Gamamabs, Genentech, GlaxoSmithKline, H3 Biomedicine, Hoffmann La Roche Ag, Imcheck Therapeutics, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Janssen Cilag, Janssen Research Foundation, Kyowa Kirin Pharm. Dev, Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncopeptides, Orion Pharma, Oxford Therapeutics, Ose Pharma, Pfizer, Pharma Mar, PEP-therapy, Pierre Fabre, Medicament, Roche, Sanofi Aventis, Sotio A.S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Xencor. SPV: Preclinical research funding: IMCore Hoffman La-Roche; AstraZeneca for work unrelated to this manuscript; Advisory board: Daiichi-Sankyo, Amgen. MA Travel: Sandoz ; Recherche grant : Sandoz ; Advisory : Viatris. DP: Consulting, advisory role or lectures: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche, Samsung; Honoraria: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche, Samsung; Clinical trials research as principal or co-investigator (Institutional financial interests): AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Pfizer, Roche, Medimmun, Sanofi-Aventis, Taiho Pharma, Novocure, Daiichi Sankyo; Travel, Accommodation, Expenses: AstraZeneca, Roche, Novartis, prIME Oncology, Pfizer. MD: MD: Consultant or Advisory Role: Astra-Zeneca, Boehringer Ingelheim, Janssen Cilag, MSD, Pfizer, Roche, Sanofi, Takeda. Speaking: Astra-Zeneca, BMS, MSD, Pfizer, Roche, Takeda. MP: MP: Consulting, advisory role or lectures: AstraZeneca, Bristol-Myers Squibb (BMS), Merck, Novartis, Pfizer, Travel, Accommodation, Expenses: AstraZeneca, Roche, Novartis,BMS. ALM: No conflicts of interest related to this study. Other conflicts of interest: - Consultant or advisory board: Amgen, Boehringer. Speaking: BMS, MSD, Pierre Fabre, Astra Zeneca. TM: Dr. Moran declares consulting/advisory role fees from Roche, Bristol Myers, Boeringher, Astra Zeneca, Lilly, and Research funding from Kyowa Kirin and Janssen, all of them unrelated with the present project. JCS is a full time-employee at Amgen since August 2021. He owns stock at Gritstone Bio, Relay Therapeutics and Amgen. BB: Contracted / supported research grants: Abbvie, Amgen, AstraZeneca, BeiGene, Blueprint Medicines, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Ignyta, IPSEN, Inivata, Janssen, Merck KGaA, MSD, Nektar, Onxeo, OSE immunotherapeutics, Pfizer, Pharma Mar, Roche-Genentech, Sanofi, Servier, Spectrum Pharmaceuticals, Takeda, Tiziana Pharma, Tolero Pharmaceuticals JCS: Personal fees outside of this work: Astex, AstraZeneca, Bayer, Blend Therapeutics, Boehringer-Ingelheim, Clovis, Eli Lilly, Gammamabs, Merus, Mission Therapeutics, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Symphogen, Tarveda; Gritstone; AstraZeneca All other authors declare no conflicts of interest.
Ethics approval and consent to participate
PIPSeN was granted central approval by the French Regulatory Authority ANSM under the reference 150127A12, on 10 April 2015, and Ethics Committee CPP Ile de France 8 on 10 Feb 2015. In Spain, PIPSeN was approved by the Agencia Española del Medicamento y Productos Sanitarios under the reference MUH/AEC, and the EC of Hospital Germans Trias i Pujol. Each enrolled patient provided informed consent as described in the manuscript and Supplementary Materials.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Postel-Vinay, S., Coves, J., Texier, M. et al. Olaparib maintenance versus placebo in platinum-sensitive non-small cell lung cancer: the Phase 2 randomized PIPSeN trial. Br J Cancer 130, 417–424 (2024). https://doi.org/10.1038/s41416-023-02514-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02514-5