Abstract
Background
The tumour-draining lymph node (TDLN) plays a pivotal role in the suppression of malignant tumour, however, the immunological profile and prognostic differences between large TDLN (L-TDLN) and small TDLN (S-TDLN) in colorectal cancer (CRC) remain unclear.
Methods
We conducted a study using data from the Chinese National Cancer Center (CNCC) database, identifying 837 CRC patients with non-metastatic TDLN, and categorised them into L-TDLN and S-TDLN groups. The long-term survival outcomes and adjuvant therapy efficacy were compared between the two groups. Furthermore, we evaluated the differences in immune activation status and immune cell subsets between patients in L-TDLN and S-TDLN groups by RNA sequencing and immunohistochemical (IHC) staining.
Results
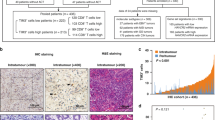
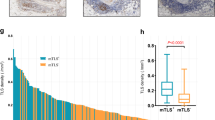
Patients with L-TDLN demonstrated better long-term outcomes compared to those with S-TDLN. Among patients with L-TDLN, there was no significant difference in long-term outcomes between those who received adjuvant chemotherapy and those who did not. The RNA sequencing data revealed a wealth of immune-activating pathways explored in L-TDLN. Furthermore, IHC analysis demonstrated higher numbers of CD3+ and CD8 + T cells in L-TDLN and the corresponding CRC lesions, as compared to patients with S-TDLN.
Conclusion
Enlarged TDLN exhibited an activated anti-tumour immune profile and may serve as an indicator for favourable survival in non-metastatic CRC.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw CRC patients’ information used in this paper are available from the lead contact upon request. RNA-seq and IHC data are available from the authors on reasonable request and approval of data sharing by institutional review boards. Correspondence for materials should be addressed to the lead contact. Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. https://doi.org/10.1016/j.immuni.2013.07.012.
Förster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 2012;33:271–80. https://doi.org/10.1016/j.it.2012.02.007.
Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T, et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell. 2020;38:685–700.e688. https://doi.org/10.1016/j.ccell.2020.09.001.
van Pul KM, Vuylsteke R, van de Ven R, Te Velde EA, Rutgers EJT, van den Tol PM, et al. Selectively hampered activation of lymph node-resident dendritic cells precedes profound T cell suppression and metastatic spread in the breast cancer sentinel lymph node. J Immunother Cancer. 2019;7:133 https://doi.org/10.1186/s40425-019-0605-1.
Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2:e284 https://doi.org/10.1371/journal.pmed.0020284.
Chen YY, Ge JY, Ma D, Yu KD. Immune-activated regional lymph nodes predict favorable survival in early-stage triple-negative breast cancer. Front Oncol. 2020;10:570981 https://doi.org/10.3389/fonc.2020.570981.
Märkl B, Paul B, Schaller T, Kretsinger H, Kriening B, Schenkirsch G. The role of lymph node size and FOXP3+ regulatory T cells in node-negative colon cancer. J Clin Pathol. 2017;70:443–7. https://doi.org/10.1136/jclinpath-2016-203978.
Märkl B, Rößle J, Arnholdt HM, Schaller T, Krammer I, Cacchi C, et al. The clinical significance of lymph node size in colon cancer. Mod Pathol. 2012;25:1413–22. https://doi.org/10.1038/modpathol.2012.92.
Hanke T, Melling N, Simon R, Sauter G, Bokemeyer C, Lebok P, et al. High intratumoral FOXP3+ T regulatory cell (Tregs) density is an independent good prognosticator in nodal negative colorectal cancer. Int J Clin Exp Pathol. 2015;8:8227–35.
Holm JB, Humphrys MS, Robinson CK, Settles ML, Ott S, Fu L, et al. Ultrahigh-throughput multiplexing and sequencing of >500-base-pair amplicon regions on the Illumina HiSeq 2500 platform. mSystems. 2019;4. https://doi.org/10.1128/msystems.00029-19.
Bao Y, Wang L, Shi L, Yun F, Liu X, Chen Y, et al. Transcriptome profiling revealed multiple genes and ECM-receptor interaction pathways that may be associated with breast cancer. Cell Mol Biol Lett. 2019;24:38 https://doi.org/10.1186/s11658-019-0162-0.
Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinforma. 2013;14:7 https://doi.org/10.1186/1471-2105-14-7.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7. https://doi.org/10.1038/nmeth.3337.
Wei R, Li S, Yu G, Guan X, Liu H, Quan J, et al. Deciphering the pyroptosis-related prognostic signature and immune cell infiltration characteristics of colon cancer. Front Genet. 2021;12:755384 https://doi.org/10.3389/fgene.2021.755384.
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–w514. https://doi.org/10.1093/nar/gkaa407.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–e110. https://doi.org/10.1158/0008-5472.can-17-0307.
Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174 https://doi.org/10.1186/s13059-016-1028-7.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. https://doi.org/10.1136/bmj.39335.541782.ad.
Huynh KT, Bilchik AJ. Sentinel lymph node biopsy and nodal ultrastaging in colorectal cancer. Cancer J. 2015;21:11–16. https://doi.org/10.1097/ppo.0000000000000093.
Chen Y, Wen Z, Ma Y, Liu Y, Que Y, Yang X, et al. Metastatic lymph node calcification in rectal cancer: comparison of CT and high-resolution MRI. Jpn J Radiol. 2021;39:642–51. https://doi.org/10.1007/s11604-021-01108-6.
Christou N, Meyer J, Toso C, Ris F, Buchs NC. Lateral lymph node dissection for low rectal cancer: is it necessary? World J Gastroenterol. 2019;25:4294–9. https://doi.org/10.3748/wjg.v25.i31.4294.
Märkl B. Stage migration vs immunology: the lymph node count story in colon cancer. World J Gastroenterol. 2015;21:12218–33. https://doi.org/10.3748/wjg.v21.i43.12218.
Inamori K, Togashi Y, Fukuoka S, Akagi K, Ogasawara K, Irie T, et al. Importance of lymph node immune responses in MSI-H/dMMR colorectal cancer. JCI Insight 2021;6. https://doi.org/10.1172/jci.insight.137365.
Piersiala K, Farrajota Neves da Silva P, Hjalmarsson E, Kolev A, Kågedal Å, Starkhammar M, et al. CD4(+) and CD8(+) T cells in sentinel nodes exhibit distinct pattern of PD-1, CD69, and HLA-DR expression compared to tumor tissue in oral squamous cell carcinoma. Cancer Sci. 2021;112:1048–59. https://doi.org/10.1111/cas.14816.
Sato D, Shimoyamada M, Ito Y, Yoshida S, Ishida H, Yamaguchi T, et al. [Reactive swelling of the regional lymph nodes in patients with stage II colorectal cancer as a prognostic factor]. Nihon Shokakibyo Gakkai Zasshi. 2017;114:1629–38. https://doi.org/10.11405/nisshoshi.114.1629.
Ruisch JE, Kloft M, Fazzi GE, Melenhorst J, Magee DR, Grabsch HI. Large negative lymph nodes - a surrogate for immune activation in rectal cancer patients? Pathol Res Pr. 2020;216:153106 https://doi.org/10.1016/j.prp.2020.153106.
Deschoolmeester V, Baay M, Lardon F, Pauwels P, Peeters M. Immune cells in colorectal cancer: prognostic relevance and role of MSI. Cancer Microenviron. 2011;4:377–92. https://doi.org/10.1007/s12307-011-0068-5.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. https://doi.org/10.1126/science.aaa1348.
van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82. https://doi.org/10.1016/s1470-2045(11)70097-3.
Bregni G, Akin Telli T, Camera S, Deleporte A, Moretti L, Bali AM, et al. Adjuvant chemotherapy for rectal cancer: current evidence and recommendations for clinical practice. Cancer Treat Rev. 2020;83:101948 https://doi.org/10.1016/j.ctrv.2019.101948.
Jiang D, Gao T, Liang S, Mu W, Fu S, Liu Y, et al. Lymph node delivery strategy enables the activation of cytotoxic T lymphocytes and natural killer cells to augment cancer immunotherapy. ACS Appl Mater Interfaces. 2021;13:22213–24. https://doi.org/10.1021/acsami.1c03709.
Buckowitz A, Knaebel HP, Benner A, Bläker H, Gebert J, Kienle P, et al. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92:1746–53. https://doi.org/10.1038/sj.bjc.6602534.
Hong EK, Chalabi M, Landolfi F, Castagnoli F, Park SJ, Sikorska K, et al. Colon cancer CT staging according to mismatch repair status: comparison and suggestion of imaging features for high-risk colon cancer. Eur J Cancer. 2022;174:165–75. https://doi.org/10.1016/j.ejca.2022.06.060.
Stintzing S, Wirapati P, Lenz HJ, Neureiter D, Fischer von Weikersthal L, Decker T, et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann Oncol. 2019;30:1796–803. https://doi.org/10.1093/annonc/mdz387.
Acknowledgements
The authors have not acknowledgement to make.
Funding
This study was supported by the National Key R&D Program for Young Scientists (Grant Number: 2022YFC2505700), the National Natural Science Foundation of China (Grant Number: 82100598), Beijing Hope Run Special Fund of Cancer Foundation of China (LC2021A23) and the Sanming Project of Medicine in Shenzhen (Grant Number: No. SZSM201911012).
Author information
Authors and Affiliations
Contributions
Conceptualisation: XSW, SMZ, ZXZ, ZJ and XG; methodology: XG, PC and RW.; formal analysis: XG, PC and RW; resources: XG, PC, RW, JTL, SJ, ZXZ, HPC and ZL; writing—original draft: XG, PC and RW; writing—review and editing: XG, PC and RW; funding acquisition: XSW, SMZ, ZXZ, ZJ and XG; supervision: XSW, SMZ, ZXZ, ZJ and XG.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study has been approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (Approval ID: 23/139–3881). The access to and use of SEER data did not require informed patient consent.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guan, X., Cheng, P., Wei, R. et al. Enlarged tumour-draining lymph node with immune-activated profile predict favourable survival in non-metastatic colorectal cancer. Br J Cancer 130, 31–42 (2024). https://doi.org/10.1038/s41416-023-02473-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02473-x