Abstract
Although PD-1/PD-L1 immunotherapy has been used successfully in treating many cancers, metastatic colorectal cancer (CRC) patients are not as responsive. B7-H3 is a promising target for immunotherapy and we found it to have the highest expression among B7-CD28 family members in CRC. Thus, the aim of the present study was to investigate B7-H3 expression in a large CRC cohort. B7-H3, B7-H4, and PD-L1 protein levels and differential lymphocyte infiltration were evaluated in tissue microarrays from 805 primary tumors and matched metastases. The relationships between immune markers, patient characteristics, and survival outcomes were determined. B7-H3 (50.9%) was detected in more primary tumors than B7-H4 (29.1%) or PD-L1 (29.2%), and elevated B7-H3 expression was associated with advanced overall stage. Co-expression of B7-H3 only with B7-H4 or PD-L1 was infrequent in primary tumors (6.3%, 5.7%, respectively). Moreover, B7-H3 in primary tumors was positively correlated with their respective expression at metastatic sites (ρ = 0.631; p < 0.001). No significant relationships between B7-H4 and PD-L1 and survival were observed; however, B7-H3 overexpression in primary tumors was significantly related to decreased disease-free survival. A positive relationship between B7-H3 expression and high density CD45RO T cell was observed in primary tumors, whereas B7-H4 and PD-L1 overexpression were related to CD3 T-cell infiltration. In conclusion, compared with B7-H4 and PD-L1, B7-H3 expression exhibited a higher prevalence and was significantly related to aggressiveness, worse prognosis and CD45RO T-cell infiltration in primary tumors. Further exploration of this potential target of immunotherapy in CRC patients is warranted.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) still presents a great burden on human health. As reported by GLOBOCAN 2018, the morbidity and mortality rates of CRC in both sexes rank third and second, respectively [1]. In China, the burden of CRC exhibits a continuously increasing trend. Approximately 370 thousand new CRC cases and 180 thousand CRC deaths occurred in 2014 [2]. Despite the prevalence and promotion of CRC screening, about a quarter of patients are diagnosed with synchronous metastases and ~50% of cases suffer from metastases during long-term follow-up [3,4,5]. For metastatic CRC (mCRC), the use of multimodality therapy, which includes surgery, chemotherapy, and ablative methods, has improved survival [3]. However, at present mCRC prognosis is poor, with a 5-year survival of 12–14% [4]. Thus, there is an urgent need for new therapies to treat mCRC patients.
Immunotherapy, targeting the PD-1/PD-L1 pathway to normalize the immune response in the tumor microenvironment, is a key treatment modality in refractory solid cancers, such as mCRC [6,7,8,9]. Two PD-1 inhibitors, pembrolizumab and nivolumab, are used to treat deficient mismatch repair (dMMR) or microsatellite instability-high (MSI-H) mCRC, due to their durable response. Unfortunately, only 5% of mCRC are dMMR or MSI-H, and most proficient MMR (pMMR) or MSI-low (MSI-L) CRC are unresponsive to immune checkpoint inhibitors [6]. Targeting other immune checkpoints may offer more immunotherapeutic choices for pMMR or MSI-L CRC patients, who are excluded from current PD-1 inhibitor therapy.
B7-H3, also known as CD276, is a B7 superfamily member [10]. B7-H3 is expressed in lymphoid cells and inhibits T-cell-mediated immune response and natural killer cell activation, although its receptor has not been identified [11, 12]. Importantly, previous studies demonstrated B7-H3 overexpression in many cancers, including breast, pancreatic, hepatocellular, renal, lung, and CRC, but limited expression in normal tissues [13,14,15,16,17,18,19]. In general, B7-H3-positive cases are associated with aggressiveness, chemoresistance, angiogenesis, and poor overall survival [20,21,22,23,24,25,26,27]. Moreover, chimeric antigen receptor T cells that limit tumor growth by targeting B7-H3 have been shown to be effective and safe [28,29,30,31]. Thus, B7-H3 is crucial for the immune response and targeting B7-H3 is a valid strategy for cancer immunotherapy.
Although some previous studies have found the association of B7-H3 with poor prognosis in CRC [32,33,34,35], the relationship between B7-H3 and alternative immune checkpoints and tumor-infiltrating immune cells is unclear. Furthermore, the concordant expression of B7-H3 between primary and matched metastatic sites has yet to be investigated. Thus, an extensive tissue microarray (TMA) CRC cohort, including 805 primary tumors and the respective metastatic sites, was designed to analyze the relationship of B7-H3, B7-H4, and PD-L1 with clinical outcomes and tumor-infiltrating immune cells.
Materials and methods
Patient cohort of China National Cancer Center and follow-up
Immunohistochemistry (IHC) data were obtained from 805 CRC patients with unselected and non-consecutive CRC that were treated from 2010 to 2014 at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The patient inclusion criteria included: (1) pathologically confirmed colorectal adenocarcinoma; (2) completed radical (R0) dissection of both primary and metastatic tumors; (3) received no preoperative anti-cancer treatments. Considering that tumor cells were rarely seen in the TMAs to evaluate because of lots of mucus, patients diagnosed with mucinous and signet-ring cell carcinomas were excluded from this study. Meanwhile, patients with multiple primary tumors or with a history of other malignancies or recurrence or without complete follow-up data or enough pathological tissue to construct the TMAs were excluded. All enrolled patients gave written or oral informed consent. Approval for the study was given by the Institutional Review Board Committee.
Patient clinicopathological variables, including age, sex, primary tumor site, tumor differentiation, TNM stage, MMR status, and postoperative treatments were retrospectively recorded. After radical surgery, all stage III and IV, and some stage II patients with high risk factors (such as T4, poor differentiation, lymphovascular invasion, preoperative intestinal obstruction etc.) received adjuvant treatment. Generally, XELOX regimen was used in colon cancer, and long course chemoradiotherapy plus XELOX regimen was used in rectal cancer. Patients were followed up regularly according to the Chinese guidelines for CRC as described previously [36]. Recurrence and distant metastasis were determined during clinical, radiological examinations, and/or histological examinations. The time between the surgery date and tumor recurrence was termed as disease-free survival (DFS). Overall survival (OS) was measured from the surgery date to the time of death or last follow-up, which was May 1, 2019.
TMA and IHC
TMA was constructed from representative areas of primary tumors, metastatic lymph nodes, and liver metastases selected from HE-stained paraffin blocks. The punched sample with a core 1.5 mm diameter was brought into the recipient block by an automatic tissue arrayer (AutoTiss 10 C, EverBio, Taiwan, China). Each TMA consisted of a primary tumor sample and matched normal mucosa tissue from the same individual. In addition, stage III TMAs included a metastatic lymph node and matched normal lymph node. Stage IV TMAs included a liver metastasis and matched normal liver tissue. To ensure reproducibility and reduce systematic errors, another copied TMA with a core 1.0 mm diameter from two different tumor areas was made for each patient. Due to limited metastatic tissues, the copied TMA only included primary tumors.
For IHC, rabbit monoclonal antibodies against B7-H3 (D9M2L), B7-H4 (D1M8I), and PD-L1 (E1L3N) and mouse monoclonal antibodies against CD45RO (UCHL1) were purchased from Cell Signaling Technology (Danvers, MA, USA). The CD3 antibodies were purchased from ZSGS-Bio (ZA0503, Beijing, China) and CD8 antibodies were purchased from Dako (C8/144B, Denmark). An automated immunostainer (BenchMark ULTRA, Ventana Medical Systems, Inc.) was utilized for IHC (4 μm thick) following standard protocols.
Quantification of B7-H3, B7-H4, and PD-L1 protein levels and immune cell infiltration
Two pathologists, blinded to patient characteristics, independently assessed the slides. In the case of discrepant results, the slides were reviewed and a consensus was achieved between the investigators.
The staining intensity of B7-H3, B7-H4, and PD-L1 expression only in tumor cells was evaluated as negative (no staining) or positive (weak to strong staining) expression. For different staining intensity in two spots from same case, the higher score was recorded. Whole slides were scanned for CD3, CD8, and CD45RO staining by a digital slide scanner (KFBIO, China) using a 40× objective equivalent. The density (cells/mm2) of positive immune cells was calculated using image analysis (HALO Indica Labs) within the tumor compartment [37].
Definition of pMMR and dMMR
MMR status in primary and metastatic sites was determined by IHC testing for MLH-1, MSH-2, MSH-6, and PMS-2. Patients simultaneously expressing all these markers were defined as pMMR. Patients lacking expression of at least one marker were defined as dMMR. For patients with abnormal MLH-1, the BRAF-V600E mutation was determined to preclude the diagnosis of Lynch syndrome.
Statistical analysis
Correlations between B7-H3, B7-H4, and PD-L1 with other patient characteristics, including the density of immune cell infiltration, were evaluated using Chi-square tests. Spearman rank correlation coefficients were employed to calculate correlations between B7-H3, B7-H4, and PD-L1 levels in primary tumors and corresponding metastatic sites. Survival was assessed and compared with the Kaplan–Meier method and log-rank tests, respectively; and comparison of four quartiles of tumor-infiltrating lymphocytes via log-rank test was made. Univariate and multivariate analyses were conducted with a Cox regression model. p < 0.05 was deemed significantly different. Statistical analyses were two-sided and accomplished using SPSS software (Version 25.0; IBM Corp., New York, USA), and SAS statistical software (Version 9.4, SAS Institute Inc., Cary, NC). Statistical analyses of RNA sequencing data from The Cancer Genome Atlas (TCGA) database (https://genomecancer.ucsu.edu/) were conducted with R version 3.4.1 (http://www.r-project.org).
Results
B7-H3, B7-H4, and PD-L1 expression in CRC
First, analyses of mRNA for B7-CD28 family members in CRC from TCGA database were performed. Among the immune markers, B7-H3 exhibited the highest expression (Supplementary Fig. S1), indicating that B7-H3 may be a potential immunotherapy target. Then, further investigation of B7-H3 protein expression level via IHC staining was performed in our cohort. We found that B7-H3 mainly exhibited cytoplasmic/membrane staining and showed different staining intensity (Supplementary Fig. S2). Of 805 primary tumors analyzed, B7-H3 was expressed in 50.9% (410/805) of CRC, including 30.9% (249/805) weak staining, 12.4% (100/805) medium staining, and 7.6% (61/805) strong staining. For positive cases, 97.6% (400/410) of samples showed positive staining in 100% of tumor cells in the core, and positive rates between two different cores correlated significantly (ρ = 0.963; p < 0.001). Meanwhile, we randomly selected ten positive and negative cases to assess B7-H3 expression in whole tissue sections, respectively, and found that the positive rates in whole tissue sections were consistent with those in TMAs (Supplementary Fig. S3), indicating homogeneous expression of B7-H3 in CRC. Also, we assessed B7-H3 expression in normal tissues, and the positive rate in normal liver (42.4%) was higher compared with normal mucosa (21.4%) and normal lymph node (11.5%) (Supplementary Fig. S4).
The fractions of patients with B7-H4 and PD-L1 positive primary tumors were 29.1% (234/805) and 29.2% (235/805), respectively. Weak staining for B7-H4 and PD-L1 was observed in 20.9% (168/805) and 20.6% (166/805), medium staining in 6.5% (52/805) and 6% (48/805), and strong staining in 1.7% (14/805) and 2.6% (21/805) of cases, respectively. The fraction of B7-H3 positive cases that only co-stained with B7-H4 or PD-L1 was low (6.3% and 5.7%, respectively) (Supplementary Fig. S5 and Supplementary Table S1).
Available tissue samples of metastatic sites were collected. Totally, 226 lymph node metastases from stage III CRC and 131 liver metastases from stage IV CRC were identified. All patients had only one corresponding metastasis to make IHC staining.
Of the 357 cases analyzed, 53.8% (192/357) of patients had B7-H3 positive primary tumors and 45.4% (162/357) were positive in metastases. Despite discordant B7-H3 expression in primary tumors versus metastases was identified in 19% (68/357) of patients, B7-H3 protein in primary tumors was moderately correlated with metastases, and the p value was statistically significant (ρ = 0.631; p < 0.001). Also, both B7-H4 and PD-L1 expression in primary tumors and metastases correlated moderately, although discordant expression in primary tumors versus metastases was identified in over 16% of patients, as shown in Table 1 (Supplementary Fig. S6).
B7-H3, B7-H4, and PD-L1 expression and clinicopathological characteristics
Correlations between clinicopathological characteristics and B7-H3, B7-H4, and PD-L1 protein levels were analyzed in 805 primary tumors, as shown in Table 2. We found that 42.7% (146/342) of stage I–II and 57% (264/463) of stage III-IV CRC cases were B7-H3 positive (p < 0.001). No other significant differences in the association of B7-H3 with clinicopathological characteristics were observed. The associations of B7-H4 and PD-L1 positive primary tumors with clinicopathological characteristics were also investigated. B7-H4 positive tumors were associated with advanced overall stage (p = 0.004), and PD-L1 positive primary tumors were associated with dMMR (p = 0.023).
In addition, significant clinicopathological differences between triple negative and triple positive primary tumors were observed. We found that triple positive cases were associated with advanced overall stage (Supplementary Table S2).
Prognostic significances of B7-H3, B7-H4, and PD-L1
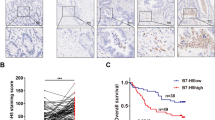
Table 3 shows the Cox regression analyses for DFS and OS. Univariate analyses found that poor tumor differentiation, stage III-IV, pMMR, adjuvant treatment and positive B7-H3 expression in primary tumors (Fig. 1a, c) were significantly associated with decreased DFS and OS (p < 0.05). Multivariate analyses indicated that B7-H3 expression in primary tumors was an independent prognostic indicator for DFS, but failed to stratify OS. Given the fact that B7-H3 was significantly correlated to TNM stage, it was not clear whether advanced TNM stage or B7-H3 caused the poor prognosis. Thus, we re-assessed the survival curves after adjustment of TNM stage via Makuch-Ghali method [38], showing that B7-H3 expression was still significantly associated with worse DFS, but not OS (Fig. 1b, d).
However, B7-H4 or PD-L1 positive primary tumors were not significantly related to DFS and OS (Supplementary Fig. S7).
Tumor-infiltrating lymphocytes and their associations with B7-H3, B7-H4, and PD-L1 expression
We calculated CD3, CD8, and CD45RO T-cell densities (cells/mm2) in whole TMA tissues using the HALO analysis system on 764, 735, and 769 primary tumors, respectively. T-cell subset densities were as follows: CD3, mean 894, median 555, interquartile range (IQR) 237–1165; CD8, mean 224, median 115, IQR 49-278; CD45RO, mean 714, median 426, IQR 176-974.
As shown in Table 4, B7-H3 positive primary tumors were significantly related to a higher CD45RO T-cell density (p = 0.001). CD3 and CD8 T-cell densities were not significantly related to B7-H3 expression. On the other hand, both PD-L1 and B7-H4 positive primary tumors were significantly related to a higher density of CD3 T-cell (p < 0.001 and p = 0.001, respectively). PD-L1 or B7-H4 expression did not exhibit significant relationships with CD8 and CD45RO T-cell densities. These data suggest that B7-H3, B7-H4, and PD-L1 positive tumors have different functions in regulating immune responses in patients with CRC.
We also assessed the prognostic significance of T-cell subset densities in CRC. Kaplan–Meier analyses without adjustment of TNM stage indicated that higher CD3, CD8, and CD45RO T-cell densities were favorable prognostic predictors of DFS and OS (Fig. 2).
Discussion
In the current study, we found that B7-H3 showed high expression at mRNA and protein levels, indicating that targeting B7-H3 may be a valid strategy for immunotherapy in CRC. In our study, B7-H3 protein expression was lower than published reports demonstrating expression ranging from 54.3% to 86% [16, 32, 33, 35, 39, 40]. In addition, we observed mainly B7-H3 cytoplasmic/membrane staining, without obvious nuclear staining, possibly due to different analytical methods, antibodies, and sample sizes with distinct races.
In addition to tumor cells, we found B7-H3 expression in normal tissues, especially in endothelial cells of the liver, which was similar to previous studies [17, 20, 28]. Detection of B7-H3 expression in normal tissues raises concerns about toxicity. However, two antibodies targeting B7-H3, MGA271 and 8H9, predominantly bind to tumor cells, with limited recognition of normal tissues, demonstrating the safety and effectiveness of targeting B7-H3 in clinical trials [41,42,43,44]. More recently, antibody-drug conjugates and chimeric antigen receptor T cells directed at B7-H3 displayed meaningful anti-tumor activity without obvious toxicity [20, 29]. These results indicate that antibodies, preferentially binding to tumor cells, can be safely used in B7-H3-overxepressing CRC.
Several studies have found that B7-H3 mainly has an inhibitory function in tumor sites and creates an immunosuppressive microenvironment with other immune checkpoints [45]. However, co-expression of B7-H3 and B7-H4 or PD-L1 at the protein level was relatively low, which was similar to the results in lung cancer [18, 19]. These data indicate that some CRC may use only one immune suppression pathway. Therefore, it may be useful to explore multi-checkpoint blockade in CRC patients who are unresponsive or resistant to PD-1/PD-L1 inhibitors. Anti-B7-H3 mAbs in solid tumors showed promise in a phase I study. Furthermore, several ongoing studies are focused on the safety and effectiveness of combination therapy with B7-H3 and PD-1/CTLA-4 inhibitors [43, 46, 47].
Positive associations between primary and metastatic site expression of B7-H3, B7-H4, and PD-L1 were observed, although discordant expression was detected in over 16% of cases. In contrast, Galon et al. showed that primary and metastases tumor cells presented a heterogeneous immune environment [48]. Moreover, many studies showed that immune checkpoints, mainly PD-L1, were more commonly expressed in metastases than primary sites, including in CRC patients [49, 50]. Recently, Kudo et al. demonstrated that brain metastases were associated with an immunosuppressed microenvironment and more tumor-associated macrophages than corresponding primary sites in non-small cell lung cancer, based on immune profiling and immune gene sequencing analysis of 39 cases [51]. However, in a study by Szekely et al., metastatic breast cancers were associated with an immune inert environment, including lower tumor-infiltrating lymphocyte counts, lower immune therapeutic targets, and lower immunotherapy response signatures, compared with primary tumors [52]. Therefore, whether metastases from primary sites are more immunosuppressive in CRC remains unclear, and future work should be conducted to fully explore the genomic and immune expression features in both primary tumors and metastatic sites.
Differential T cells in the tumor microenvironment are crucial in the immune response. Thus, we evaluated CD3, CD8, and CD45RO T-cell densities associated with B7-H3, B7-H4, and PD-L1 expression. We showed a significant relationship between elevated CD3, CD8, and CD45RO T-cell densities and improved prognosis, similar to previous studies [53]. In addition, we noticed that tumor cell B7-H3, B7-H4, and PD-L1 protein levels were associated with differential lymphocyte infiltration, indicating that immune checkpoint effects on T cells vary substantially. Although B7-H3 was originally shown to positively affect T-cell activation, recent studies demonstrated a potential B7-H3 inhibitory function during T-cell activation, and B7-H3 blockade increased CD8 T-cell infiltration and promoted functional recovery of cytotoxic T cells [10,11,12, 45, 54]. However, we found a positive correlation between B7-H3 and CD45RO T-cell density, but not with CD3 and CD8 T-cell densities. CD45RO, one isoform of CD45, is crucial in immune cell activation. Previous studies showed that after T-cell activation, CD45RO expression replaced the silenced CD45RA, referred to as naive human T cells [55, 56]. CD45RO T cell may regulate the immune response to tumor cells via activation of T cells. However, whether this immune activation is further inhibited by B7-H3 overexpression remains unclear, and future work should be conducted to identify its receptor to explore the mechanism.
Previous studies suggest that dMMR tumors are associated with an active immune environment, a high level of immune cell infiltration, tumor PD-L1 overexpression, and favorable prognosis compared with pMMR tumors [57,58,59]. In our study, we also observed a positive correlation of dMMR status with favorable prognosis and PD-L1 overexpression. This may partially explain why cases that were PD-L1 positive had a higher density of CD3 T-cell in the current study. However, B7-H3 expression and MMR status had no significant association. This suggests that anti-B7-H3 therapy may be applicable to more patients with CRC, and not only those with dMMR tumors.
B7-H4 is believed to be a negative regulator of the immune response and is positively associated with the infiltration depth and metastasis [60,61,62]. In the current study, we found that B7-H4 expression correlates with high CD3 T-cell density and advanced overall stage, indicating that B7-H4 may be involved in metastasis. Future work should focus on the function of B7-H4 in CRC.
Although a meta-analysis conducted by Fan et al. suggested that B7-H3 expression in CRC was associated with poor survival, B7-H3 expression in different cell sites had different prognostic significance [34]. Only studies conducted by Ingebrigsten et al. found that B7-H3 was expressed in the nucleus and nuclear B7-H3 was an independent poor prognostic factor, while the prognostic significance of nuclear B7-H3 was not further confirmed by their latter results [32, 39]. Most studies indicated that B7-H3 was mainly expressed in the cytoplasm/membrane of tumor cells, with variable impact on survival [16, 32, 33, 35, 39, 40]. These discrepant results could have been because of differences in antibodies, methodologies, and sample sizes. In the current study, we used the validated antibody (B7-H3, D9M2L) and a large cohort, finding that B7-H3 expression was mainly detected in cytoplasm/membrane of tumor cells and was independently associated with decreased DFS, but not OS, which indicated that CRC patients expressing B7-H3 are more likely to experience relapses.
Our study had several limitations. First, it was a retrospective study, enrolling Chinese patients from a single-center setting, which might cause selection bias. However, the large sample size would make our findings more generalizable. Second, only colorectal adenocarcinoma was included in this study, and other tumor subtypes, such as mucinous and signet-ring cell carcinoma, were excluded, which might lack representativeness. Thus, relationships between B7-H3 expression and other histological subtypes should be investigated in the future. Third, we used TMAs to explore the immune checkpoints and lymphocyte infiltration expression. The results might lack representation because of tumor heterogeneity. However, increasing studies measuring immune checkpoints and lymphocyte infiltration expression in different tumors using TMAs showed consistent results, demonstrating the reliability of this approach [18, 53, 63, 64]. Moreover, we used two different tumor cores to explore B7-H3 expression and the positive rates in randomly selected whole tissue sections were consistent with those in TMAs, which showed homogeneous expression, supporting the reproducibility of our findings. Fourth, although we performed an extensive analysis of immune markers in metastases, all primary tumors had only one matched metastasis. Several studies showed tumor heterogeneity from different metastases [48, 65], therefore all available metastases should be collected to make analyses in the future. In addition, although patients at high risk for recurrence received adjuvant therapy, different cytotoxic regimens and cycles might be used. Last, different antibodies and analysis methods used in our study make it difficult to compare with previous results.
In conclusion, compared with B7-H4 and PD-L1, B7-H3 expression exhibited a higher prevalence and co-expression of B7-H3 with B7-H4 and PD-L1 was infrequent in primary tumors. In addition, B7-H3 expression in primary tumors correlated with their corresponding expression in metastatic sites. Moreover, B7-H3 expression in primary tumors was significantly related to advanced overall stage, worse prognosis, and CD45RO T-cell infiltration, supporting B7-H3 as a potential immunotherapeutic target in CRC patients.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12.
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii1–9.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93.
Benitez Majano S, Di Girolamo C, Rachet B, Maringe C, Guren MG, Glimelius B, et al. Surgical treatment and survival from colorectal cancer in Denmark, England, Norway, and Sweden: a population-based study. Lancet Oncol. 2019;20:74–87.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–91.
Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–26.
Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74.
Lee YH, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034–45.
Yonesaka K, Haratani K, Takamura S, Sakai H, Kato R, Takegawa N, et al. B7-H3 negatively modulates CTL-mediated cancer immunity. Clin Cancer Res. 2018;24:2653–64.
Arigami T, Narita N, Mizuno R, Nguyen L, Ye X, Chung A, et al. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann Surg. 2010;252:1044–51.
Inamura K, Takazawa Y, Inoue Y, Yokouchi Y, Kobayashi M, Saiura A, et al. Tumor B7-H3 (CD276) expression and survival in pancreatic cancer. J Clin Med. 2018;7.
Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–7.
Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, et al. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163–71.
Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi Y, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61:2171–82.
Altan M, Pelekanou V, Schalper KA, Toki M, Gaule P, Syrigos K, et al. B7-H3 expression in NSCLC and its association with B7-H4, PD-L1 and tumor-infiltrating lymphocytes. Clin Cancer Res. 2017;23:5202–9.
Carvajal-Hausdorf D, Altan M, Velcheti V, Gettinger SN, Herbst RS, Rimm DL, et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human small cell lung Cancer (SCLC). J Immunother Cancer. 2019;7:65.
Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell. 2017;31:501–15.e8.
Zhang C, Zhang Z, Li F, Shen Z, Qiao Y, Li L, et al. Large-scale analysis reveals the specific clinical and immune features of B7-H3 in glioma. Oncoimmunology. 2018;7:e1461304.
Aung PP, Parra ER, Barua S, Sui D, Ning J, Mino B, et al. B7-H3 expression in merkel cell carcinoma-associated endothelial cells correlates with locally aggressive primary tumor features and increased vascular density. Clin Cancer Res. 2019;25:3455–67.
Lin L, Cao L, Liu Y, Wang K, Zhang X, Qin X, et al. B7-H3 promotes multiple myeloma cell survival and proliferation by ROS-dependent activation of Src/STAT3 and c-Cbl-mediated degradation of SOCS3. Leukemia. 2019;33:1475–86.
Liu Z, Zhang W, Phillips JB, Arora R, McClellan S, Li J, et al. Immunoregulatory protein B7-H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene. 2019;38:88–102.
Purvis IJ, Avilala J, Guda MR, Venkataraman S, Vibhakar R, Tsung AJ, et al. Role of MYC-miR-29-B7-H3 in medulloblastoma growth and angiogenesis. J Clin Med. 2019;8:1158.
Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F, et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10:308.
Son Y, Kwon SM, Cho JY. CD276 (B7-H3) maintains proliferation and regulates differentiation in angiogenic function in late endothelial progenitor cells. Stem Cells. 2019;37:382–94.
Du H, Hirabayashi K, Ahn S, Kren NP, Montgomery SA, Wang X, et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell. 2019;35:221–37.e8.
Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25:2560–74.
Nehama D, Di Ianni N, Musio S, Du H, Patane M, Pollo B, et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine. 2019;47:33–43.
Tang X, Zhao S, Zhang Y, Wang Y, Zhang Z, Yang M, et al. B7-H3 as a novel CAR-T therapeutic target for glioblastoma. Mol Ther Oncolytics. 2019;14:279–87.
Ingebrigtsen VA, Boye K, Tekle C, Nesland JM, Flatmark K, Fodstad O. B7-H3 expression in colorectal cancer: nuclear localization strongly predicts poor outcome in colon cancer. Int J Cancer. 2012;131:2528–36.
Bin Z, Guangbo Z, Yan G, Huan Z, Desheng L, Xueguang Z. Overexpression of B7-H3 in CD133+ colorectal cancer cells is associated with cancer progression and survival in human patients. J Surg Res. 2014;188:396–403.
Fan H, Zhu JH, Yao XQ. Prognostic significance of B7-H3 expression in patients with colorectal cancer: a meta-analysis. Pak J Med Sci. 2016;32:1568–73.
Zhang T, Wang F, Wu JY, Qiu ZC, Wang Y, Liu F, et al. Clinical correlation of B7-H3 and B3GALT4 with the prognosis of colorectal cancer. World J Gastroenterol. 2018;24:3538–46.
Lu Z, Cheng P, Zhang MG, Wang XS, Zheng ZX. Is adjuvant chemotherapy necessary for patients with ypT0-2N0 rectal cancer treated with neoadjuvant chemoradiotherapy and curative surgery? Gastroenterol Rep. 2018;6:277–83.
Danilova L, Ho WJ, Zhu Q, Vithayathil T, De Jesus-Acosta A, Azad NS, et al. Programmed cell death ligand-1 (PD-L1) and CD8 expression profiling identify an immunologic subtype of pancreatic ductal adenocarcinomas with favorable survival. Cancer Immunol Res. 2019;7:886–95.
Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis. 1982;35:437–43.
Ingebrigtsen VA, Boye K, Nesland JM, Nesbakken A, Flatmark K, Fodstad O. B7-H3 expression in colorectal cancer: associations with clinicopathological parameters and patient outcome. BMC Cancer. 2014;14:602.
Liu X, Wang F, Wu J, Zhang T, Liu F, Mao Y, et al. Expression of CYP1B1 and B7-H3 significantly correlates with poor prognosis in colorectal cancer patients. Int J Clin Exp Pathol. 2018;11:2654–64.
Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834–45.
Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61:4048–54.
Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409–18.
Kramer K, Smith M, Souweidane MM. Safety profile of long-term intraventricular access devices in pediatric patients receiving radioimmunotherapy for central nervous system malignancies. Pediatr Blood Cancer. 2014;61:1590–2.
Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906.
Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo HF, et al. Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7-H3. J Biol Chem. 2015;290:30018–29.
Benzon B, Zhao SG, Haffner MC, Takhar M, Erho N, Yousefi K, et al. Correlation of B7-H3 with androgen receptor, immune pathways and poor outcome in prostate cancer: an expression-based analysis. Prostate Cancer Prostatic Dis. 2017;20:28–35.
Van den Eynde M, Mlecnik B, Bindea G, Fredriksen T, Church SE, Lafontaine L, et al. The Link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell. 2018;34:1012–26.e3.
Wang HB, Yao H, Li CS, Liang LX, Zhang Y, Chen YX, et al. Rise of PD-L1 expression during metastasis of colorectal cancer: Implications for immunotherapy. J Dig Dis. 2017;18:574–81.
Garcia-Diez I, Hernandez-Ruiz E, Andrades E, Gimeno J, Ferrandiz-Pulido C, Yebenes M, et al. PD-L1 expression is increased in metastasizing squamous cell carcinomas and their metastases. Am J Dermatopathol. 2018;40:647–54.
Kudo Y, Haymaker C, Zhang J, Reuben A, Duose DY, Fujimoto J, et al. Suppressed immune microenvironment and repertoire in brain metastases from patients with resected non-small-cell lung cancer. Ann Oncol. 2019;30:1521–30.
Szekely B, Bossuyt V, Li X, Wali VB, Patwardhan GA, Frederick C, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29:2232–9.
Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–66.
Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–6.
Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–8.
Rudd CE, Anderson P, Morimoto C, Streuli M, Schlossman SF. Molecular interactions, T-cell subsets and a role of the CD4/CD8:p56lck complex in human T-cell activation. Immunol Rev. 1989;111:225–66.
Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417–22.
Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51.
Inaguma S, Lasota J, Wang Z, Felisiak-Golabek A, Ikeda H, Miettinen M. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Mod Pathol. 2017;30:278–85.
Ichikawa M, Chen L. Role of B7-H1 and B7-H4 molecules in down-regulating effector phase of T-cell immunity: novel cancer escaping mechanisms. Front Biosci. 2005;10:2856–60.
Zhao LW, Li C, Zhang RL, Xue HG, Zhang FX, Zhang F, et al. B7-H1 and B7-H4 expression in colorectal carcinoma: correlation with tumor FOXP3(+) regulatory T-cell infiltration. Acta Histochem. 2014;116:1163–8.
Li C, Zhan Y, Ma X, Fang H, Gai X. B7-H4 facilitates proliferation and metastasis of colorectal carcinoma cell through PI3K/Akt/mTOR signaling pathway. Clin Exp Med. 2020;20:79–86.
Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66.
Schalper KA, Carvajal-Hausdorf D, McLaughlin J, Altan M, Velcheti V, Gaule P, et al. Differential expression and significance of PD-L1, IDO-1, and B7-H4 in human lung cancer. Clin Cancer Res. 2017;23:370–8.
Jimenez-Sanchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell. 2017;170:927–38.e20.
Acknowledgements
We specially thank Xiao-Shuang Feng for statistical analysis. This work was also supported by National Key R&D Program of China (2017YFC0908203), CAMS Initiative for Innovative Medicine (2017-I2M-2-003, 2016-I2M-1-001, and 2018-I2M-AI-008), Science and Technology Project of Chaoyang District, Beijing (CYSF-1931), and Beijing Science and Technology Program (D17110002617004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, Z., Zhao, ZX., Cheng, P. et al. B7-H3 immune checkpoint expression is a poor prognostic factor in colorectal carcinoma. Mod Pathol 33, 2330–2340 (2020). https://doi.org/10.1038/s41379-020-0587-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0587-z
This article is cited by
-
Incorporating IL7 receptor alpha signaling in the endodomain of B7H3-targeting chimeric antigen receptor T cells mediates antitumor activity in glioblastoma
Cancer Immunology, Immunotherapy (2024)
-
B7-H3 at the crossroads between tumor plasticity and colorectal cancer progression: a potential target for therapeutic intervention
Cancer and Metastasis Reviews (2024)
-
Soluble B7-H3 in Colorectal Cancer
Bulletin of Experimental Biology and Medicine (2023)
-
Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy
Journal of Hematology & Oncology (2022)
-
Expression of B7 family checkpoint proteins in cervical cancer
Modern Pathology (2022)