Abstract
Background
Previously suggested modifiable risk factors for prostate cancer could have resulted from detection bias because diagnosis requires a biopsy. We investigated modifiable risk factors for a subsequent cancer diagnosis among men with an initially negative prostate biopsy.
Methods
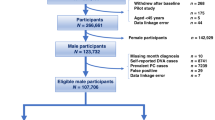
In total, 10,396 participants of the Health Professionals Follow-up Study with an initial negative prostate biopsy after 1994 were followed for incident prostate cancer until 2017. Potential risk factors were based on previous studies in the general population. Outcomes included localised, advanced, and lethal prostate cancer.
Results
With 1851 prostate cancer cases (168 lethal) diagnosed over 23 years of follow-up, the 20-year risk of any prostate cancer diagnosis was 18.5% (95% CI: 17.7–19.3). Higher BMI and lower alcohol intake tended to be associated with lower rates of localised disease. Coffee, lycopene intake and statin use tended to be associated with lower rates of lethal prostate cancer. Results for other risk factors were less precise but compatible with and of similar direction as for men in the overall cohort.
Conclusions
Risk factors for future prostate cancer among men with a negative biopsy were generally consistent with those for the general population, supporting their validity given reduced detection bias, and could be actionable, if confirmed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Information regarding procedures for obtaining and accessing HPFS data is described at https://www.hsph.harvard.edu/hpfs/for-collaborators/.
Code availability
All code is available in conjunction with the study data.
References
Kilpeläinen TP, Tammela TL, Roobol M, Hugosson J, Ciatto S, Nelen V, et al. False-positive screening results in the European randomized study of screening for prostate cancer. Eur J Cancer. 2011;47:2698–705.
Gann PH, Fought A, Deaton R, Catalona WJ, Vonesh E. Risk factors for prostate cancer detection after a negative biopsy: a novel multivariable longitudinal approach. J Clin Oncol. 2010;28:1714–20.
Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. J Am Med Assoc. 2018;319:1901–13.
Kilpeläinen TP, Tammela TL, Määttänen L, Kujala P, Stenman UH, Ala-Opas M, et al. False-positive screening results in the Finnish prostate cancer screening trial. Br J Cancer. 2010;102:469–74.
Lewicki P, Shoag J, Golombos DM, Oromendia C, Ballman KV, Halpern JA, et al. Prognostic significance of a negative prostate biopsy: an analysis of subjects enrolled in a prostate cancer screening trial. J Urol. 2017;197:1014–9.
Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, Culig Z, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484–92.
Tangen CM, Goodman PJ, Till C, Schenk JM, Lucia MS, Thompson IM Jr. Biases in recommendations for and acceptance of prostate biopsy significantly affect assessment of prostate cancer risk factors: results from two large randomized clinical trials. J Clin Oncol. 2016;34:4338–44.
Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. A prospective cohort study of vasectomy and prostate cancer in US men. J Am Med Assoc. 1993;269:873–7.
Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–8.
Genkinger JM, Wu K, Wang M, Albanes D, Black A, van den Brandt PA, et al. Measures of body fatness and height in early and mid-to-late adulthood and prostate cancer: risk and mortality in The Pooling Project of Prospective Studies of Diet and Cancer. Ann Oncol. 2020;31:103–14.
Pernar CH, Ebot EM, Pettersson A, Graff RE, Giunchi F, Ahearn TU, et al. A prospective study of the association between physical activity and risk of prostate cancer defined by clinical features and TMPRSS2:ERG. Eur Urol. 2019;76:33–40.
Graff RE, Pettersson A, Lis RT, Ahearn TU, Markt SC, Wilson KM, et al. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. 2016;103:851–60.
Rowles JL 3rd, Ranard KM, Applegate CC, Jeon S, An R, Erdman JW Jr. Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose-response meta-analysis. Prostate Cancer Prostatic Dis. 2018;21:319–36.
Chen X, Zhao Y, Tao Z, Wang K. Coffee consumption and risk of prostate cancer: a systematic review and meta-analysis. BMJ Open. 2021;11:e038902.
Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC, et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2015;101:87–117.
Bylsma LC, Alexander DD. A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr J. 2015;14:125.
Knuppel A, Papier K, Fensom GK, Appleby PN, Schmidt JA, Tong TYN, et al. Meat intake and cancer risk: prospective analyses in UK Biobank. Int J Epidemiol. 2020;49:1540–52.
Lee KH, Seong HJ, Kim G, Jeong GH, Kim JY, Park H, et al. Consumption of fish and ω-3 fatty acids and cancer risk: an umbrella review of meta-analyses of observational studies. Adv Nutr. 2020;11:1134–49.
Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100:693–701.
Allott EH, Ebot EM, Stopsack KH, Gonzalez-Feliciano AG, Markt SC, Wilson KM, et al. Statin use is associated with lower risk of PTEN-null and lethal prostate cancer. Clin Cancer Res. 2020;26:1086–93.
Sauer CM, Myran DT, Costentin CE, Zwisler G, Safder T, Papatheodorou S, et al. Effect of long term aspirin use on the incidence of prostate cancer: a systematic review and meta-analysis. Crit Rev Oncol/Hematol. 2018;132:66–75.
Hong S, Khil H, Lee DH, Keum N, Giovannucci EL. Alcohol consumption and the risk of prostate cancer: a dose-response meta-analysis. Nutrients. 2020;12:2188.
Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Alcoholic beverages and prostate cancer in a prospective US cohort study. Am J Epidemiol. 2010;172:773–80.
Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26.
Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80.
Pernar CH, Chomistek AK, Barnett JB, Ivey K, Al-Shaar L, Roberts SB, et al. Validity and relative validity of alternative methods to assess physical activity in epidemiologic studies: findings from the men’s lifestyle validation study. Am J Epidemiol. 2022;191:1307–22.
Graff RE, Ahearn TU, Pettersson A, Ebot EM, Gerke T, Penney KL, et al. Height, obesity, and the risk of TMPRSS2:ERG-defined prostate cancer. Cancer Epidemiol, Biomark Prev. 2018;27:193–200.
Downer MK, Kenfield SA, Stampfer MJ, Wilson KM, Dickerman BA, Giovannucci EL, et al. Alcohol intake and risk of lethal prostate cancer in the health professionals follow-up study. J Clin Oncol. 2019;37:1499–511.
Stopsack KH, Gonzalez-Feliciano AG, Peisch SF, Downer MK, Gage RA, Finn S, et al. A prospective study of aspirin use and prostate cancer risk by TMPRSS2:ERG status. Cancer Epidemiol Biomark Prev. 2018;27:1231–3.
Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–8.
Wilson KM, Shui IM, Mucci LA, Giovannucci E. Calcium and phosphorus intake and prostate cancer risk: a 24-y follow-up study. Am J Clin Nutr. 2015;101:173–83.
Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC, et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomark Prev. 2003;12:64–67.
Richman EL, Kenfield SA, Stampfer MJ, Giovannucci EL, Chan JM. Egg, red meat, and poultry intake and risk of lethal prostate cancer in the prostate-specific antigen-era: incidence and survival. Cancer Prev Res. 2011;4:2110–21.
Giovannucci E, Liu Y, Stampfer MJ, Willett WC. A prospective study of calcium intake and incident and fatal prostate cancer. Cancer Epidemiol Biomark Prev. 2006;15:203–10.
Michaud DS, Augustsson K, Rimm EB, Stampfer MJ, Willet WC, Giovannucci E. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control CCC. 2001;12:557–67.
Wilson KM, Kasperzyk JL, Rider JR, Kenfield S, van Dam RM, Stampfer MJ, et al. Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-up Study. J Natl Cancer Inst. 2011;103:876–84.
Hurwitz LM, Agalliu I, Albanes D, Barry KH, Berndt SI, Cai Q, et al. Recommended definitions of aggressive prostate cancer for etiologic epidemiologic research. J Natl Cancer Inst. 2021;113:727–34.
Jahn JL, Giovannucci EL, Stampfer MJ. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the prostate-specific antigen-era. Int J Cancer. 2015;137:2795–802.
Pierre-Victor D, Parnes HL, Andriole GL, Pinsky PF. Prostate cancer incidence and mortality following a negative biopsy in a population undergoing PSA screening. Urology. 2021;155:62–69.
Sayyid RK, Alibhai SMH, Sutradhar R, Eberg M, Fung K, Klaassen Z, et al. Population-based outcomes of men with a single negative prostate biopsy: Importance of continued follow-up among older patients. Urol Oncol. 2019;37:298.e219–98.e227.
Klemann N, Røder MA, Helgstrand JT, Brasso K, Toft BG, Vainer B, et al. Risk of prostate cancer diagnosis and mortality in men with a benign initial transrectal ultrasound-guided biopsy set: a population-based study. Lancet Oncol. 2017;18:221–9.
Palmstedt E, Månsson M, Frånlund M, Stranne J, Pihl CG, Hugosson J, et al. Long-term outcomes for men in a prostate screening trial with an initial benign prostate biopsy: a population-based cohort. Eur Urol Oncol. 2019;2:716–22.
Stroomberg HV, Andersen MCM, Helgstrand JT, Larsen SB, Vickers AJ, Brasso K, et al. Standardized prostate cancer incidence and mortality rates following initial non-malignant biopsy result. BJU Int. 2023;132:181–7.
Kenfield SA, Batista JL, Jahn JL, Downer MK, Van Blarigan EL, Sesso HD, et al. Development and application of a lifestyle score for prevention of lethal prostate cancer. JNCI J Natl Cancer Inst. 2015;108:djv329.
Sutcliffe S, Giovannucci E, Leitzmann MF, Rimm EB, Stampfer MJ, Willett WC, et al. A prospective cohort study of red wine consumption and risk of prostate cancer. Int J Cancer. 2007;120:1529–35.
Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL. Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988-2006. Int J Cancer. 2011;128:2444–52.
Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, Stampfer MJ, et al. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. 2014;106:djt430.
Möller E, Wilson KM, Batista JL, Mucci LA, Bälter K, Giovannucci E. Body size across the life course and prostate cancer in the Health Professionals Follow-up Study. Int J Cancer. 2016;138:853–65.
Harrison S, Tilling K, Turner EL, Lane JA, Simpkin A, Davis M, et al. Investigating the prostate specific antigen, body mass index and age relationship: is an age-BMI-adjusted PSA model clinically useful? Cancer Causes Control CCC. 2016;27:1465–74.
Wallner LP, Morgenstern H, McGree ME, Jacobson DJ, St Sauver JL, Jacobsen SJ, et al. The effects of body mass index on changes in prostate-specific antigen levels and prostate volume over 15 years of follow-up: implications for prostate cancer detection. Cancer Epidemiol Biomark Prev. 2011;20:501–8.
Al-Azab R, Toi A, Lockwood G, Kulkarni GS, Fleshner N. Prostate volume is strongest predictor of cancer diagnosis at transrectal ultrasound-guided prostate biopsy with prostate-specific antigen values between 2.0 and 9.0 ng/mL. Urology. 2007;69:103–7.
Chiam K, Bang A, Patel MI, Nair-Shalliker V, O’Connell DL, Smith DP. Characteristics associated with the use of diagnostic prostate biopsy and biopsy outcomes in Australian men. Cancer Epidemiol Biomark Prev. 2021;30:1735–43.
Acknowledgements
We thank the participants and staff of the HPFS for their valuable contributions. The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centres. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming.
Funding
This study was supported by the Zhu Family Center for Global Cancer Prevention and by the US National Cancer Institute (U01 CA167552, R03 CA226942). PLN, KLP, LAM and KHS are Prostate Cancer Foundation Young Investigators. ELG is funded as an American Cancer Society Clinical Research Professor (grant CRP-23-1014041).
Author information
Authors and Affiliations
Contributions
LAM and KHS conceived the study idea and designed the study. XF, YZ, JBV, RL, ELG, LAM and KHS contributed to the methodology. XF, YZ, JBV and RL contributed to the data extraction. XF, YZ, and RL performed the statistical analysis. XF, YZ, PLN, KLP, ELG, LAM and KHS interpreted the analysis. XF and KHS drafted the manuscript. All authors critically revised the manuscript. LAM and KHS supervised the project. All authors gave final approval for the version to be submitted. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
KLP reports research grants/funding from Janssen. PLN reports research funding from Astellas, Bayer, and Janssen; consulting for Nanocan, Boston Scientific, Bayer, Janssen, Novartis, and Blue Earth; and equity in Nanocan. LAM reports grants/funding from Bayer, AstraZeneca, and Janssen; has provided expert testimony for Bayer. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the institutional review board at the Harvard T.H. Chan School of Public Health, and participating cancer registries, as required.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, X., Zhang, Y., Vaselkiv, J.B. et al. Modifiable risk factors for subsequent lethal prostate cancer among men with an initially negative prostate biopsy. Br J Cancer 129, 1988–2002 (2023). https://doi.org/10.1038/s41416-023-02472-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02472-y