Abstract
Background
Short-term infusions of dinutuximab beta plus isotretinoin and cytokines administered in previous immunotherapy studies in neuroblastoma were associated with severe pain. Here, long-term, continuous infusion of single-agent dinutuximab beta was evaluated in patients with relapsed/refractory neuroblastoma.
Methods
In this open-label, single-arm, Phase 2 study, patients with either refractory or relapsed high-risk neuroblastoma received dinutuximab beta by continuous infusion over 10 days of each cycle, for up to five cycles. The primary endpoint was objective response rate 24 weeks after the end of cycle 5. Secondary endpoints included adverse events, intravenous morphine use, best response, duration of response, and three-year progression-free and overall survival.
Results
Of the 40 patients included, 38 had evaluable response. Objective response rate was 26% and best response rate 37%. Median duration of response was 238 days (IQR 108–290). Three-year progression-free and overall survival rates were 31% (95% CI 17–47) and 66% (95% CI 47–79), respectively. Prophylactic intravenous morphine use and duration of use decreased with increasing cycles. The most common grade 3 treatment-related adverse events were pain, diarrhea, and hypokalemia.
Conclusion
Long-term continuous infusion of single-agent dinutuximab beta is tolerable and associated with clinically meaningful responses in patients with relapsed/refractory high-risk neuroblastoma.
Clinical trial registration
The study is registered with ClinicalTrials.gov (NCT02743429) and EudraCT (2014-000588-42).
Similar content being viewed by others
Background
Neuroblastoma is the most common extracranial solid tumor of childhood and accounts for 12–15% of cancer-related deaths in children [1]. Approximately 50% of patients with neuroblastoma have high-risk disease [2], associated with three-year event-free survival (EFS) of only ~50%, despite intensive multimodal treatment [3, 4].
A randomized trial has demonstrated that therapy with an anti-GD2 antibody, interleukin-2 (IL-2), granulocyte-macrophage colony-stimulating factor (GM-CSF) and isotretinoin in the maintenance setting improves outcomes of patients with high-risk neuroblastoma (HR-NB) [5]. However, as GD2 is not only expressed on neuroblastoma cells, but also on sensory neuronal tissue [6], a common on-target side effect of anti-GD2 antibodies is neuropathic pain, which requires the co-administration of analgesic drugs, including intravenous morphine [7]. Another on-target side effect associated with anti-GD2 antibodies is neurotoxicity, which also requires careful management with supportive therapy [8].
A long-term, continuous infusion regimen for the anti-GD2 antibody dinutuximab beta was developed based on the clinical observation that decreasing the speed of the infusion reduced the incidence and severity of pain. In a single-center compassionate use program in patients with HR-NB, dinutuximab beta (100 mg/m2/cycle) infused continuously over 10 days combined with IL-2 and isotretinoin was associated with an acceptable safety profile, including low pain scores, a reduced need for intravenous morphine, and a low frequency of grade 3 and grade 4 adverse events [9].
In the HR-NBL1/SIOPEN study, where patients with HR-NB were given dinutuximab beta as a short, 8-h infusion with or without IL-2 in addition to isotretinoin, co-administration of IL-2 was found to increase toxicity, without further improving outcomes [10].
Differentiation therapy with isotretinoin has been the standard of care for patients with neuroblastoma for many years, based on the results of a randomized clinical study demonstrating its benefits on EFS [11]. However, long-term follow-up data indicated that isotretinoin had no significant effect on either EFS or overall survival (OS) over the longer term [12].
Here, we report the results of a multicenter clinical study investigating the activity and safety of single-agent dinutuximab beta (i.e., without IL-2 or isotretinoin) administered by long-term, continuous infusion in patients with relapsed/refractory HR-NB.
Methods
Study design and participants
This was a prospective, open-label, single-arm, multicenter, Phase 2 study (APN311-304) in which single-agent dinutuximab beta, administered by continuous, long-term infusion, was investigated in patients with relapsed/refractory HR-NB. Patients aged 1–21 years with neuroblastoma, according to the International Neuroblastoma Staging System (INSS) criteria [13], were eligible for inclusion if they had primary refractory Stage 4 disease or had relapsed after primary Stage 4 disease, or had developed distant metastases following primarily localized neuroblastoma, and their tumor burden was controlled using conventional therapy but with measurable disease still present. Patients who received previous anti-GD2 antibody therapies were eligible, if they were negative for anti-dinutuximab beta antibody prior to study entry.
Procedures
Dinutuximab beta 10 mg/m2/day was administered as continuous infusion over the first 10 days of each 35-day cycle (Supplementary Fig. S1) for up to five cycles (in the absence of disease progression). Dinutuximab beta was planned to be administered in the hospital setting in each cycle, but if well tolerated, it could be given in an outpatient setting from day 5 of cycle 1.
Pain management with oral gabapentin, and morphine was administered according to the World Health Organization guidelines. Based on the individual patient’s pain tolerance, subsequent treatment days within a cycle as well as subsequent treatment cycles could be started with an adapted intravenous morphine dose. Prophylaxis for fever was metamizole, acetaminophen, or ibuprofen, according to institutional standards. All patients received hydration during administration of dinutuximab beta to prevent hypotension.
Chemotherapy, radiotherapy, hormonal anticancer therapy, or experimental anticancer medications were not permitted during the study or the 12- and 24-week follow-up periods. The use of intravenous immunoglobulin was discouraged during dinutuximab beta treatment.
Outcomes
The primary endpoint of the study was the objective response rate (ORR) 24 weeks after the end of cycle 5, defined as the proportion of patients with a complete or partial response based on the International Neuroblastoma Response Criteria (INRC) [13]. The secondary endpoints were duration of response, three-year progression-free survival (PFS) and OS rates, response at each tumor evaluation, best response, safety and tolerability, pharmacokinetics, pharmacodynamics, and immunogenicity.
Tumor assessments according to the INRC, including magnetic resonance imaging (MRI), computed tomography (CT) and 123I-metaiodobenzylguanidine (mIBG) scan, were undertaken in the last 2 weeks of cycle 2, at the end of cycle 5 (or at the end of treatment if discontinued before cycle 5), 12 and 24 weeks after completing study treatment, and then every three months for 3 years. All assessments were centrally reviewed by a blinded review committee.
Safety and tolerability assessments included changes in vital signs and clinical laboratory parameters, pain intensity assessment scores (0 [no pain] to 10 [unbearable pain] on a validated pain scale), and the need for, amount, and duration of intravenous morphine. In addition, adverse events were recorded and graded using the Common Terminology Criteria for Adverse Events, version 4.0.
Pharmacokinetic assessments included determining dinutuximab beta serum concentrations in cycle 1 at baseline, the end of infusion, and on days 15, 22, 29, and 35 [14]. Pharmacodynamic assessments included antibody-dependent cell-mediated cytotoxicity, and complement-dependent cytotoxicity [15]. The sampling time points for pharmacokinetic and pharmacodynamic parameters are shown in Supplementary Fig. S1. Immunogenicity was assessed by the presence of human anti-chimeric antibodies (HACA) [16, 17].
Statistical analysis
For the primary endpoint, a sample size of 40 evaluable patients would provide at least 80% power to reject the null hypothesis of an ORR of <10% if the true ORR observed was 25%, which is ~20% higher than the estimated historical response rate [18, 19] in this patient population (overall one-sided significance level 0.05, exact binomial test).
The primary endpoint was analyzed using the exact binomial test (one-sided) using SAS Version 9.4, with an overall significance level of 0.05, corresponding to an alpha of 0.1 for a two-sided test. Post-hoc analyses of the primary endpoint were also undertaken: (1) in patients with bone marrow involvement, (2) in patients with relapsed versus refractory disease, and (3) based on an updated INRC definition of ORR that includes minor response [20]. Fisher’s exact test (two-sided) was used to determine if there was a significant association between disease status at study entry and response.
Survival probabilities were estimated using Kaplan–Meier methods, and statistical comparisons were performed using the log-rank test (assuming proportional hazard). Three-year survival rates are shown as means and 95% confidence interval (CI). A post-hoc analysis was conducted to evaluate PFS and OS in patients stratified by disease status at study entry (relapsed versus refractory disease).
Pharmacokinetic, pharmacodynamic and immunogenicity data are presented as mean and standard deviation (SD). Differences between groups were calculated using a Mann–Whitney rank sum test [21].
Results
Between March 30, 2015, and December 7, 2018, 40 patients with relapsed or refractory HR-NB were recruited, all of whom received dinutuximab beta and were included in the safety analysis set. Baseline characteristics are shown in Table 1. Tumor responses were evaluated in 38 patients (full analysis set): 21 with relapsed and 17 with refractory disease. Three patients received previous anti-GD2 antibody therapy.
Cycle 1 of dinutuximab beta was completed by 39 patients (mean cumulative dose per cycle: 91.5 mg/m2 [SD 15.7]). The number of patients receiving subsequent cycles of dinutuximab beta decreased due to progressive disease. Patients were hospitalized for the first 7 days (median, interquartile range [IQR] 6–10) of cycle 1, reducing to 3 days (median, IQR 3–3.25) in cycle 5 (Fig. 1a), indicating that an increasing proportion of the 10-day continuous infusion could be administered in an outpatient setting.
The duration of hospitalization for antibody treatment (a), proportion of patients requiring intravenous morphine during five treatment cycles with single-agent dinutuximab beta (b) and the daily dose of intravenous morphine required (c). a The box limits are the 1st and 3rd quartile with the median value indicated by the red line and the whiskers represent the most extreme points (1.5 × IQR); outliers are not displayed. + indicates the mean value. The duration of hospitalization was calculated as the date of discharge-date of admission +1. If readmission occurs within 11 days of day 1 of the cycle, the date of readmission is added. b The data represent the mean and in panel c the mean ± SD. DB dinutuximab beta, IQR interquartile range, i.v. intravenous, SD standard deviation.
All patients received intravenous morphine on day 1 of cycle 1 at a mean dose of 0.611 mg/kg (SD 0.191) (Fig. 1b). The dose of morphine required decreased within the cycles, and with subsequent cycles (Fig. 1c). The mean duration of morphine treatment also generally decreased with subsequent cycles, from 3.2 days (SD 1.4) in cycle 1, to 2.2 days (SD 0.9), and 0.3 days (SD 0.8) in cycles 2 and 3, respectively, and 0 days in cycles 4–5.
During cycle 1, 92% of patients reported pain at rest, compared with ~22% during cycle 5 (Supplementary Table S1). In patients who experienced pain, mean pain scores were low and decreased between cycles 1 and 2: from 1.01 to 0.30 at rest, and from 1.45 to 0.28 at stress.
Overall, 13 (32%) patients who received dinutuximab beta had treatment-related grade 3 toxicities (Table 2); no grade 4 or 5 events were reported. The most common grade 3 toxicities were neuropathic pain, diarrhea, and hypokalemia. No cases of grade 3 hypotension, capillary leak syndrome, hypersensitivity, bronchospasm, allergic reaction, or neurotoxicity were reported.
The ORR 24 weeks after the end of cycle 5 of dinutuximab beta was 26% (10/38), which was higher than the null hypothesis of 10% (P = 0.0034). When minor responses were also considered, the ORR was 32% (12/38). Nine responding patients were refractory and three had relapsed disease, indicating a statistically significant association between response rate and disease status at study entry (P = 0.0159). The ORR based on the best overall response up to 24 weeks after the end of cycle 5 was 37% (14/38, P < 0.0001), and 53% (20/38) when minor responses were also considered (Table 3 and Supplementary Table S2). In the post-hoc analysis of the 14 patients with bone marrow involvement at baseline, 12 patients achieved a complete response and one a partial response, indicating a response rate of 93%. In the full analysis set, the median duration of response was 238.0 days (IQR 108–290). The best response rate according to the INRC components varied between assessments and was highest in the bone marrow compartment (93%) followed by the osteomedullary compartment assessed using mIBG scans (53%) and the soft tissue compartment assessed by CT/MRT scans (17%) (Supplementary Tables S3–5).
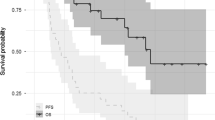
The three-year PFS rate was 31% (95% CI 0.17–0.47) and OS rate was 66% (95% CI 0.47–0.79) in the full analysis set (n = 38) (Fig. 2). When patients were analysed according to their disease status at study entry, three-year survival rates were lower for patients with relapsed disease compared with those with refractory disease (three-year PFS rate: 19% [95% CI 2–36] vs 47% [95% CI 21–73, P = 0.015]; three-year OS rate: 50% [95% CI 28–72] vs 93% [95% CI 81–1, P = 0.015]) (Fig. 2).
In total, 35 patients were evaluable for the pharmacokinetic analysis—one patient was excluded due to missing samples, and two developed HACAs during cycle 1. The concentration-time curve for dinutuximab beta administered as long-term infusion (Fig. 3a) showed a mean peak serum concentration at the end of infusion of 11.2 µg/ml (SD 3.2) during cycle 1, followed by rapid clearance with an alpha half-life of 2.3 days (SD 2.1) and a terminal beta half-life of 7.8 days (SD 2.9).
Plasma concentration-time curve for dinutuximab beta administered by long-term, continuous infusion over 10 days (a). Pharmacodynamic effects of dinutuximab beta on complement-dependent cytotoxicity in cycles 1, 3 and 5 (b) and antibody-dependent cellular cytotoxicity in cycle 1 (c). Cumulative incidence of human anti-chimeric antibody response after dinutuximab beta treatment (d). a–c Data represent mean ± SD. All P values are versus values on day 1 of cycle 1 using the Mann–Whitney rank sum test: *P < 0.0001; #P = 0.0057; ‡P < 0.0001; §P = 0.0208.
In the pharmacodynamic analysis (n = 35), serum levels of dinutuximab beta translated into significantly increased complement-dependent (11-fold; Fig. 3b) and antibody-dependent cellular cytotoxicity (seven-fold; Fig. 3c) on day 8 in cycle 1 compared with pre-treatment (day 1) in cycle 1 (Fig. 3b). The complement activity was significantly increased at trough dinutuximab beta levels preceding subsequent cycles (day 1 of cycles 3 and 5 compared with pre-treatment [day 1] in cycle 1; Fig. 3b), indicating immunological activity in patients treated with dinutuximab beta over the duration of the treatment period. HACA response at the end of treatment was found in eight of 37 (22%) patients, and the cumulative incidence over time shows that the response occurred during the first two cycles. There was no correlation between the dinutuximab beta peak or trough concentration, or the area under the curve in cycle 1, and HACA response or survival (data not shown).
Discussion
This open-label, Phase 2 study evaluated long-term continuous infusion of dinutuximab beta without IL-2 or isotretinoin in patients with relapsed/refractory HR-NB. The best overall response rate (37%), three-year PFS rate (31%), three-year OS rate (66%), and median duration of response (238 days) indicate the clinical activity and efficacy of single-agent dinutuximab beta. Interestingly, the response and survival rates in patients with refractory disease were signifcantly higher than in patients with relapsed disease, suggesting that future trials should be stratified by separating these subgroups.
Response and survival are similar to those achieved when dinutuximab beta was administered in combination with IL-2 and isotretinoin in a similar patient population (ORR 41%, three-year PFS rate 33%, three-year OS rate 48%) [9], suggesting no additional benefit for IL-2 and isotretinoin co-administration. However, ideally, a randomized trial should be conducted comparing dinutuximab beta with or without IL-2 and isotretinoin in this population, similar to the front-line setting [10]. Similar tumor responses were also reported with the humanized anti-GD2 antibody naxitamab co-administered with GM-CSF in a Phase 1/2 study in patients with primary or secondary refractory neuroblastoma [22]. In an interim analysis including 38 patients, the ORR was 34% and the duration of response was at least 6 months [22], which is similar to the findings of the current study in the absence of concomitant cytokines. In another Phase 2 study in patients with HR-NB with bone and/or bone marrow disease, naxitamab plus GM-CSF resulted in an ORR of 68% [23]. In a subgroup of 14 patients with bone marrow involvement in the current study, the response rate was 93%, suggesting that anti-GD2 immunotherapy has particularly high activity against bone marrow disease.
Based on this study, single-agent dinutuximab beta administered by long-term infusion is associated with a better safety profile than that reported in a study evaluating dinutuximab beta combined with IL-2 [9]. This is true for both the on-target side effects of pain and neurotoxicity, as well as inflammatory side effects. The use of intravenous morphine was not required by any patient during treatment cycles 4 and 5 of single-agent dinutuximab beta; in contrast, it was necessary in all five cycles when dinutuximab beta was combined with IL-2 [9]. Given the observation that >90% of patients did not require intravenous morphine after cycle 2 in our study, it is likely that >90% of patients could receive treatment in the outpatient setting from cycle 3 onwards. In a similar study evaluating the use of dinutuximab beta combined with isotretinoin, the proportion of patients who did not require intravenous morphine after cycle 2 was found to be 100% [24]. The incidence of grade 3 pain observed in this study (18%) was lower than for dinutuximab beta plus IL-2 in previous studies (26–38%) [9, 10]. Furthermore, there were no cases of grade 3 and 4 capillary leak syndrome, hypersensitivity reactions, bronchospasm, or allergic reactions with single-agent dinutuximab beta, while in studies where it was co-administered with IL-2, the incidences were 13–15%, 2–21%, 8%, and 15%, respectively [9, 10].
The pharmacokinetics of dinutuximab beta were similar when administered alone here or co-administered with IL-2 in an earlier study, with mean peak plasma concentration of 11.2 µg/ml (SD 3.2) and 12.56 µg/ml (SD 0.68), respectively [17]. Similarly, the incidence of HACA responses and the levels of complement-dependent and antibody-dependent cellular cytotoxicity responses were also comparable with or without IL-2 [25]. These results demonstrate that there is no loss of biological activity in the absence of IL-2. The absence of a correlation between the peak/trough concentration or the area under the curve of dinutuximab beta in cycle 1, and HACA response or survival is in contrast to previous reports for dinutuximab [26, 27], underlining the molecular differences between these antibodies.
Limitations of the study include the heterogeneity of the enrolled patient population regarding their second-line therapy. The study was conducted to determine the activity of single-agent dinutuximab beta in patients with relapsed/refractory disease who had responded to a second-line therapy that was not predefined in the study protocol. Therefore, as all patients had to be in stable disease to be eligible, the responses must be interpreted with caution. In contrast, many other Phase 2 studies in neuroblastoma included patients with progressive disease at study entry.
Despite these limitations, our findings demonstrate that single-agent dinutuximab beta (i.e., without IL-2 and isotretinoin) administered by long-term, continuous infusion in patients with relapsed/refractory HR-NB was associated with improved tolerability and clinically significant and durable response rates that translated into encouraging three-year PFS and OS rates.
Data availability
The data generated in this study are available upon request from the corresponding author.
References
Johnsen, JI, Dyberg, C, Wickström, M. Neuroblastoma—a neural crest derived embryonal malignancy. Front Mol Neurosci. 2019;12:9.
DuBois SG, Macy ME, Henderson TO. High-risk and relapsed neuroblastoma: toward more cures and better outcomes. Am Soc Clin Oncol Educ Book. 2022;42:1–13.
Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. J Am Med Assoc. 2019;322:746–55.
Garaventa A, Poetschger U, Valteau-Couanet D, Luksch R, Castel V, Elliot M, et al. Randomized trial of two induction therapy regimens for high-risk neuroblastoma: HR-NBL1.5 International Society of Pediatric Oncology European Neuroblastoma Group Study. J Clin Oncol. 2021;39:2552–63.
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34.
Doronin II, Vishnyakova PA, Kholodenko IV, Ponomarev ED, Ryazantsev DY, Molotkovskaya IM, et al. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer. 2014;14:295.
Barone G, Barry A, Bautista F, Brichard B, Defrachelles AS, Herd F, et al. Managing adverse events associated with dinutuximab beta treatment in patients with high-risk neuroblastoma: practical guidance. Paediatr Drugs. 2021;23:537–48.
Wieczorek A, Manzitti C, Garaventa A, Gray J, Papadakis V, Valteau-Couanet D, et al. Clinical phenotype and management of severe neurotoxicity observed in patients with neuroblastoma treated with dinutuximab beta in clinical trials. Cancers. 2022;14:1919.
Mueller I, Ehlert K, Endres S, Pill L, Siebert N, Kietz S, et al. Tolerability, response and outcome of high-risk neuroblastoma patients treated with long-term infusion of anti-GD(2) antibody ch14.18/CHO. MAbs. 2018;10:55–61.
Ladenstein R, Pötschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1617–29.
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–73.
Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27:1007–13.
Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–77.
Siebert N, Seidel D, Eger C, Brackrock D, Reker D, Schmidt M, et al. Validated detection of anti-GD2 antibody ch14.18/CHO in serum of neuroblastoma patients using anti-idiotype antibody ganglidiomab. J Immunol Methods. 2013;398–399:51–9.
Siebert N, Seidel D, Eger C, Jüttner M, Lode HL. Functional bioassays for immune monitoring of high-risk neuroblastoma patients treated with ch14.18/CHO anti-GD2 antibody. PLoS ONE. 2014;9:e107692.
Siebert N, Eger C, Seidel D, Jüttner M, Lode HL. Validated detection of human anti-chimeric immune responses in serum of neuroblastoma patients treated with ch14.18/CHO. J Immunol Methods. 2014;407:108–15.
Siebert N, Eger C, Seidel D, Jüttner M, Zumpe M, Wegner D, et al. Pharmacokinetics and pharmacodynamics of ch14.18/CHO in relapsed/refractory high-risk neuroblastoma patients treated by long-term infusion in combination with IL-2. MAbs. 2016;8:604–16.
Modak S, Kushner BH, Basu E, Roberts SS, Cheung NV. Combination of bevacizumab, irinotecan, and temozolomide for refractory or relapsed neuroblastoma: results of a phase II study. Pediatr Blood Cancer. 2017;64:e26448.
Mody R, Naranjo A, Van Ryn C, Yu AL, London WB, Shulkin BL, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18:946–57.
Park JR, Bagatell R, Cohn SL, Pearson AD, Villablanca JG, Berthold F, et al. Revisions to the international neuroblastoma response criteria: a consensus statement from the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2017;35:2580–7.
Perme MP, Manevski D. Confidence intervals for the Mann-Whitney test. Stat Methods Med Res. 2019;28:3755–68.
Y-mAbs Therapeutics Inc. Danyelza® (naxitamab-gqgk) US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761171lbl.pdf. Accessed 24 January 2023 (2020).
Mora J, Chan G, Morgenstern D, Nysom K, Bear M, Dalby LW, et al. Efficacy and updated safety results from pivotal phase II trial 201 of naxitamab (Hu3F8), a humanized GD2 targeted immunotherapy for the treatment of refractory/relapsed (R/R) high risk (HR) neuroblastoma (NB). Ann Oncol. 2020;31:S7.
Cicek F, Troschke-Meurer S, Ceylan K, Jahns LJ, Zumpe M, Siebert N, et al. Impact of IL-2 on treatment tolerance in patients with high-risk neuroblastoma treated with dinutuximab beta-based immunotherapy. Front Pediatr. 2020;16:582820.
Siebert N, Troschke-Meurer S, Marx M, Zumpe M, Ehlert K, Gray J, et al. Impact of HACA on immunomodulation and treatment toxicity following ch14.18/CHO long-term infusion with interleukin-2: results from a SIOPEN phase 2 trial. Cancers. 2018;10:387.
Mody R, Yu AL, Naranjo A, Zhang FF, London WB, Shulkin BL, et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the Children’s Oncology Group. J Clin Oncol. 2020;38:2160–9.
Desai AV, Gilman AL, Ozkaynak MF, Naranjo A, London WB, Tenney SC, et al. Outcomes following GD2-directed postconsolidation therapy for neuroblastoma after cessation of random assignment on ANBL0032: a report from the Children’s Oncology Group. J Clin Oncol. 2022;40:4107–18.
Acknowledgements
Recloning and production of the ch14.18 monoclonal antibody was done at Polymun, Vienna, Austria, and was enabled by a SIOPEN fundraising effort in 2001. Apeiron Biologics provided the product during the study. Neither Polymun nor Apeiron Biologics had a role in the study design or the analysis. Apeiron Biologics and later EUSA Pharma (liscensee of the product as of 2017) provided funding for the clinical research organization FGK Clinical Research GmbH and Gabriele Dürr-Watzka and her team who supported with the conduct of the study, data collection and analysis. Further support for sample analysis was obtained from Hector Stiftungen (Grant M2116). The authors express their gratitude and appreciation to the treating physicians, academic clinical research and care teams, and, most importantly, the patients and families facing high-risk neuroblastoma for their committed participation in the study. The authors thank Sandra Riesebeck, Maria Asmus, Manuela Brüser, and Theodor Koepp (University Medicine Greifswald, Pediatric Hematology and Oncology, Greifswald, Germany) and Dasa Janousek, Ingrid Pribill, and Claudia Zeiner-Koglin (St. Anna Kinderkrebsforschung e.V., Children’s Cancer Research Institute, Vienna, Austria) for excellent assistance. Editorial assistance for the development of the manuscript was provided by Clare Ryles and Katrin Male of mXm Medical Communications funded by EUSA Pharma. Editorial advice was also provided by Prof Andy Pearson from the Institute of Cancer Research at the Royal Marsden Hospital, Sutton, UK. The content of the article represents the views of the authors and has not been influenced by third-party sponsorship.
Funding
This research was funded by the University Medicine Greifswald, HW and J Hector Stiftungen, Germany, under Grant M2116, Apeiron Biologics, Vienna, Austria under Grant APN, and Apeiron (Vienna, Austria) providing dinutuximab beta (ch14.18/CHO), and the St. Anna Kinderkrebsforschung (Vienna, Austria). Further funding was provided by EUSA Pharma (Hemel Hempstead, UK), which has marketing authorization for dinutuximab beta in Europe. Editorial assistance for the development of the manuscript was funded by EUSA Pharma. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
H Lode, H Loibner and RL conceived and designed the study. KE, H Lode, RL, and SH provided study materials and recruited patients. H.Lode, NS, MZ, RL, and ST-M collected data and interpreted it along with H Loibner. H Lode wrote the first draft of the manuscript. All authors reviewed, edited and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
RL and H Lode acted as consultants for Apeiron and EUSA Pharma for the clinical development of dinutuximab beta. H Lode participated in advisory boards organized by EUSA Pharma. H Loibner was CEO of Apeiron until July 2018. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in compliance with Good Clinical Practice guidelines and in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committees of the University Medicine of Greifswald, Germany, and the Paul-Ehrlich Institute in Langen, Germany. All patients or their parents/guardians provided written, informed consent before study entry.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Loibner retired in July, 2018.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lode, H.N., Ehlert, K., Huber, S. et al. Long-term, continuous infusion of single-agent dinutuximab beta for relapsed/refractory neuroblastoma: an open-label, single-arm, Phase 2 study. Br J Cancer 129, 1780–1786 (2023). https://doi.org/10.1038/s41416-023-02457-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02457-x