Abstract
Researchers and research funders aiming to improve diagnosis seek to identify if, when, where, and how earlier diagnosis is possible. This has led to the propagation of research studies using a wide range of methodologies and data sources to explore diagnostic processes. Many such studies use electronic health record data and focus on cancer diagnosis. Based on this literature, we propose a taxonomy to guide the design and support the synthesis of early diagnosis research, focusing on five key questions:
-
Do healthcare use patterns suggest earlier diagnosis could be possible?
-
How does the diagnostic process begin?
-
How do patients progress from presentation to diagnosis?
-
How long does the diagnostic process take?
-
Could anything have been done differently to reach the correct diagnosis sooner?
We define families of diagnostic research study designs addressing each of these questions and appraise their unique or complementary contributions and limitations. We identify three further questions on relationships between the families and their relevance for examining patient group inequalities, supported with examples from the cancer literature. Although exemplified through cancer as a disease model, we recognise the framework is also applicable to non-neoplastic disease. The proposed framework can guide future study design and research funding prioritisation.
Similar content being viewed by others
Introduction
Researchers and research funders increasingly recognise the imperative to improve diagnosis in medicine [1, 2]. The recent growth in research relating to diagnostic quality and safety is challenging to navigate, due to the different study designs employed to address the same research questions and the lack of consensus on terminology.
We posit that research in the field of diagnostic quality and safety aims to answer five principal questions concerning the diagnostic process:
-
1.
Do healthcare use patterns suggest earlier diagnosis could be possible?
-
2.
How does the diagnostic process begin?
-
3.
How do patients progress from presentation to diagnosis?
-
4.
How long does the diagnostic process take?
-
5.
Could anything have been done differently to reach the correct diagnosis sooner?
A taxonomy (a classification scheme) of different research questions underpinned by theoretical considerations can support a systematic approach to understanding relevant literature and can guide priorities in future research for different conditions. Thus, we discuss study designs and methods best suited to address each of these five questions. Further, we explore how examining variation between and within these study families can advance the understanding of how diagnosis can be improved across patient groups.
Five principal questions concerning the diagnostic process
Do healthcare use patterns suggest earlier diagnosis could be possible? (Diagnostic window studies)
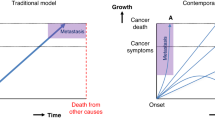
The diagnostic window is defined by a pre-diagnostic period where the frequency of healthcare encounters made by an as-yet-undiagnosed cohort (i.e., the group of patients with a pre-specified condition who present because of their underlying condition but who have not as yet received their true diagnosis) increases from ‘background’ healthcare use in the same patients or disease-free controls. The length of the diagnostic window provides a guide to how much earlier it may be possible to diagnose at least some patients with the condition.
Different types of healthcare events can define diagnostic windows, helping to elucidate when the condition becomes detectable in specific ways. Windows defined by primary care consultations provide a generic expression of when as-yet-undiagnosed patients begin to use healthcare differently. Diagnostic windows can additionally be defined by events recorded during the healthcare encounter (e.g., recorded symptoms, prescribed medication, investigations ordered [3, 4]). When conducted in different disease contexts, health systems or eras, diagnostic window studies can reveal differences in diagnostic performance and identify patient groups with the greatest potential for earlier diagnosis [5,6,7].
The unique strength of diagnostic window studies is that they provide proof-of-concept epidemiological evidence that earlier diagnosis may – in principle – be possible. Empirically demonstrating the existence and length of diagnostic windows is a useful first step in designing diagnostic research. This length not only informs the length of follow-up that should be considered in further studies, but also indicates the period during which quality improvement efforts should focus and the degree of population-level improvement that may be possible.
A principal limitation of diagnostic window studies is that they do not demonstrate the proportion of patients responsible for changes in healthcare use. In theory, a very small number of highly atypical patients could account for detectable changes. Further, diagnostic windows do not provide insight into the exact clinical circumstances of individual patients and do not produce evidence that any specific patient could have been diagnosed any earlier, unlike studies of missed diagnostic opportunities (see below).
How does the diagnostic process begin? (Prodromal feature studies)
Prodromal features are characteristics that are observed in the as-yet-undiagnosed population at a greater rate than in controls who remain disease-free. Many studies consider prodromal symptoms [8, 9], but there are other possible prodromal features such as abnormal test results [10, 11]. Analyses of large samples of electronic health records have enabled formal identification and quantification of these early signs and symptoms in recent years, alongside their positive predictive values for cancer [8, 12,13,14].
The main strength of prodromal feature studies is that known prodromal features can be used to guide the diagnostic process, for example in helping to decide whether specialist investigations or referrals are needed. There are several examples of studies estimating the predictive value of symptoms and tests supporting clinical practice guidelines [15,16,17,18,19,20].
A limitation of these studies is the variability in the length of the period during which features associated with the diagnosis are observed. Some of this variation may be appropriate as different features are likely to be predictive over different periods of time, however much literature in the field of cancer early diagnosis research uses 1- or 2-year periods a priori without justification. Formal evidence from diagnostic window studies can be useful in determining risk periods of appropriate length for studies of early signs and symptoms, particularly for conditions characterised by vague or non-specific symptoms. A new approach uses time-to-event analysis to explore how the association of a feature with diagnosis of a condition changes over time [21].
How do patients progress from presentation to diagnosis? (Diagnostic pathway studies)
Diagnostic pathways comprise the sequence of different healthcare encounters, investigations, and decisions in a patient’s journey to diagnosis. For example, in symptomatic lung cancer patients, different pathways might encompass visiting a GP with a prodromal feature, being sent for a chest X-ray, referred to a respiratory outpatient department or having an emergency admission [22].
A diagnostic pathway begins when a patient first recognises a symptom and ends when the correct diagnosis is made [23, 24]. During this pathway patients typically present to healthcare in three ways, as described in Fig. 1. A small number of additional patients who experience minimal or no prodromal features may have unheralded diagnoses, only recorded on their death certificate [25]. Variation in the proportion of patients presenting through each route may be indicators of our ability to diagnose a condition electively [26, 27].
Walter et al. note two initial patient-dependent stages of the diagnostic pathway – the “appraisal” and “help-seeking” stages [24]. These stages cannot be identified from structured electronic health record data, and require alternative approaches, such as free-text or qualitative interviews with patients and clinicians which may be more susceptible to bias [28,29,30,31]. As such, diagnostic pathway studies using EHRs will typically focus on identifying pathways from first presentation through to diagnosis.
Identifying patient-level diagnostic pathways and analysing patterns at population-level produces a map of the routes through which patients typically first present and then progress towards a final diagnosis via tests, prescriptions, and referrals. In the context of diseases with diagnostic guidelines, the proportion of patients diagnosed via guideline-concordant pathways can help assess the success of quality improvement initiatives [32, 33]. Similarly, the “optimality” of different pathways can be assessed by comparing their associations with prognosis and patient experience [34], or through clinician ranking [35].
How long does the diagnostic process take? (Diagnostic interval studies)
The diagnostic interval for an individual patient is the period between first presentation and diagnosis and is a measure of how long it takes for them to be correctly diagnosed. The ‘total’ interval, from symptom onset to diagnosis or treatment, can be further split into subcomponents, such as the patient interval and the primary care interval [28] (Fig. 2).
Considering diagnostic intervals at population-level allows identification of whether patients with the as-yet-undiagnosed condition are likely to experience diagnostic delay, and quantification of the distribution of any delays. Further, examining changes in average diagnostic intervals can support the evaluation of diagnosis improvement initiatives - such as the introduction of clinical guidelines - and can help to compare performance between and across healthcare systems [36,37,38,39,40]. However, diagnostic intervals should be triangulated with other measures of diagnostic delay to understand whether comorbidities may be artefactually prolonging interval length [7].
The main limitation of both diagnostic pathway and diagnostic interval studies is that, generally, pathways or intervals alone are not sufficient to determine if anything could have been done differently to ensure a specific patient was diagnosed sooner. However, by examining variation with other factors, they can provide an understanding of which patients are at the highest risk of experiencing diagnostic delay and where in the healthcare system delay is most likely to occur. Depending on the disease and healthcare context, these studies may require linkage of multiple datasets to track patient pathways, determine pathway optimality, or measure intervals.
Could anything have been done differently to reach the correct diagnosis sooner? (Missed diagnostic opportunity studies)
Missed diagnostic opportunities are pre-diagnosis healthcare contacts where post-hoc judgement indicates that alternative decisions or actions could have led to more timely diagnosis [41]. The majority of missed diagnostic opportunities are expected to occur within the diagnostic window and relate to patients with suboptimal diagnostic pathways and prolonged diagnostic intervals. However, there is little empirical research currently demonstrating this.
A current challenge is the unresolved balance between identifying missed diagnostic opportunities both accurately and objectively. One method of identifying missed diagnostic opportunities is manual clinical review [42,43,44], but this requires resources that limit scalability beyond clinical audit projects.
A second method is to define markers of missed diagnostic opportunities in EHR data. Such phenotypic rules – also termed ‘e-triggers’ - typically incorporate the documented occurrence of an event, combined with a time period during which a subsequent action ought to have followed [45,46,47,48,49]. This allows estimation of the prevalence of a specific missed diagnostic opportunity, but requires prior knowledge of relevant markers.
A third approach is to consider any contacts within the diagnostic window where relevant symptoms have occurred - above those expected coincidentally - as missed diagnostic opportunities [50]. This gives a proxy marker for missed opportunities and still requires manual clinical review to determine whether any individual instance was truly a missed diagnostic opportunity.
Identifying missed diagnostic opportunities can provide both patient- and population- level insight into diagnostic quality and safety incidents that are taking place and their frequency. This could allow for fast and targeted action to improve the diagnostic process. However, any approach incorporating a clinical review component may be subject to hindsight bias [51] – that is, the clinician’s awareness of the patient’s outcome may affect their judgement of whether a missed diagnostic opportunity occurred.
Discussion
The proposed taxonomy can be used to understand the diagnostic process and systematically organise existing evidence and is summarised in Table 1. We believe there are three additional questions that can be asked within these families of studies to examine why diagnostic quality and safety deviations are occurring and their potential impact.
What factors are associated with variation in a study family?
For any of the families above we can examine associations with various factors. These include patient factors (age, sex, deprivation, comorbidities, ethnicity, and whether the patient lives alone), healthcare factors (location, type, and size of the healthcare setting), disease factors (cancer morphological type and grade), and era. This can provide insight into mechanisms responsible for prolonged diagnostic delay or convoluted pathways to diagnosis and help target interventions at affected groups.
For example, variations in diagnostic interval with age, and diagnostic pathways with cancer site have been observed [36, 52]. Small differences in diagnostic window length with sex have been shown for primary intracranial tumours [53].
What relationships exist between these study families?
We may frequently have research questions that relate to multiple study families; for example, are missed diagnostic opportunities more prevalent on certain diagnostic pathways? We can also explore how variation within one family can explain variation within another alongside the factors considered above: if a particular patient group commonly take a suboptimal diagnostic pathway, is this due to the early signs and symptoms they present with, or for other reasons?
For colorectal cancer, for example, women with serious non-gastrointestinal comorbidities who have an emergency presentation have been shown to have a diagnostic window twice as long compared to other patient groups [54, 55]. This shows that for certain patient subgroups targeted improvement efforts could help diagnose patients earlier.
What impact does variation within a study family have on disease outcomes or patient experience?
We can evaluate the impact of specific diagnostic process experiences by exploring associations between disease outcomes or patient experience and the families above. This helps us to appreciate the consequences of diagnostic quality and safety lapses, and facilitates discussion of which targeted interventions may have the most impact.
Earlier diagnosis of symptomatic cancer is likely to improve survival and quality of life, although benefits vary by cancer site [56]. It has also been shown that patient experience varies with diagnostic pathway for breast, colon, and rectal cancer, with emergency presenters reporting worse and screening-detected patients reporting the greatest satisfaction with care [57].
Strengths and limitations
The proposed taxonomy explains the main research questions addressed by diagnostic quality and safety research, explores how different study families address these questions and provides a framework against which existing and new research can be organised. Further, it provides an opportunity to standardise terminology used across diagnostic quality and safety research.
A key concern is the extent to which “confounding by indication” may bias research on the diagnostic process. In brief, diagnostic management is influenced by the patient’s health status seen by a clinician [58]. The potential bias this may cause is best illustrated in diagnostic interval studies. Tørring et al. discuss a “U-shaped” relationship between diagnostic interval length and mortality in colorectal cancer patients [59] (also known as the ‘waiting time paradox’ or ‘sicker-quicker’ phenomenon [56, 60,61,62]). Counterintuitively, there was higher mortality among patients with the shortest diagnostic intervals. This is possibly explained by tumour aggressiveness and stage at presentation, emergency presentations, and multi-morbidity [59]. Concerns have also been raised as to how multi-morbidity may affect the measurement of diagnostic intervals [7]. Researchers should consider methods to assess the presence of confounding, for example comparing intervals by stage or diagnostic pathway groups.
The study families we have described focus on earlier diagnosis as a process measure (achieving diagnosis earlier in time), rather than as a patient/disease outcome (achieving diagnosis at an earlier disease stage). Whilst shorter intervals in the diagnostic process are associated with improved patient outcomes in general [56], Tørring et al. have illustrated that this association varies between patient groups [59]. For cancer, staging classifications are well-developed, but for other conditions disease stage or severity may not be well-defined. Furthermore, we have not considered research concerning overdiagnosis. At present, defining and quantifying overdiagnosis is challenging [63, 64] and generally only possible for patient groups as opposed to individual patients. Nevertheless, consideration of potential overdiagnosis is required when carrying out any of the research we have described.
In developing the taxonomy, we have focused on research using electronic health records. Other possible research methods – such as surveys or qualitative research have not been considered in detail. There may be some overlap in the purposes of the families we describe; for example, both diagnostic windows and intervals could be used to identify conditions where diagnostic delay is a concern. When designing diagnostic quality and safety research, it may be useful to consider the differences between families and how they can address specific questions being asked (Supplementary Table 1).
Finally, the examples we give to support our taxonomy were sourced from the literature on cancer diagnosis, and some of the research we have described may not be possible for certain other health conditions. For example, endometriosis diagnosis and management are entwined and cannot be separated, so defining a date of diagnosis may not always be possible. Without a diagnosis date carrying out diagnostic window studies, for example, would be very challenging.
Implications
This taxonomy provides a structure against which existing evidence can be compared and organised, helping to elucidate promising targets for further research and improvement efforts. This allows us to borrow methods and adapt findings from diagnostic quality and safety research into other, seemingly unrelated, diseases. This is particularly relevant for conditions where existing evidence may be sparse, such as schizophrenia and rheumatoid arthritis.
The proposed framework can guide research in a sequential fashion; for example, if we want to explore how a specific condition is diagnosed in a specific healthcare system, then we can methodologically work through the families distinguished here (as applicable to the condition) to build the knowledge base, from population- to patient-level.
Conclusion
We propose a ‘5-question’ taxonomy of diagnostic quality and safety research. The proposed framework can help situate existing research and deepen enquiries into diagnostic quality and safety deviations in conditions such as cancer, where diagnostic delay continues to be prevalent despite growing investment in research. It can also guide rigorous diagnostic quality and safety research in conditions for which existing evidence is sparse. This taxonomy will aid the synthesis of existing evidence, support the design of new studies, and prioritise decisions for research aiming to improve diagnosis in medicine as a whole, and for specific conditions.
References
Committee on Diagnostic Error in Health Care, Board on Health Care Services, Institute of Medicine, The National Academies of Sciences Engineering and Medicine. Improving Diagnosis in Health Care. National Academies Press (US), 2015. https://doi.org/10.17226/21794.
Crosby D, Lyons N, Greenwood E, Harrison S, Hiom S, Moffat J, et al. A roadmap for the early detection and diagnosis of cancer. Lancet Oncol. 2020;21:1397–9.
Zhou Y, Abel GA, Hamilton W, Singh H, Walter FM, Lyratzopoulos G. Imaging activity possibly signalling missed diagnostic opportunities in bladder and kidney cancer: a longitudinal data-linkage study using primary care electronic health records. Cancer Epidemiol. 2020;66:101703.
Zhou Y, Walter FM, Mounce L, Abel GA, Singh H, Hamilton W, et al. Identifying opportunities for timely diagnosis of bladder and renal cancer via abnormal blood tests: a longitudinal linked data study. Br J Gen Pr. 2021;72:e19–25.
Christensen KG, Fenger-Grøn M, Flarup KR, Vedsted P. Use of general practice, diagnostic investigations and hospital services before and after cancer diagnosis - a population-based nationwide registry study of 127,000 incident adult cancer patients. BMC Health Serv Res. 2012;12:224.
Wang Y, Freemantle N, Nazareth I, Hunt K. Gender differences in survival and the use of primary care prior to diagnosis of three cancers: an analysis of routinely collected UK general practice data. PLoS One. 2014;9:101562.
Price S, Wiering B, Mounce LTA, Hamilton W, Abel G. Examining methodology to identify patterns of consulting in primary care for different groups of patients before a diagnosis of cancer: an exemplar applied to oesophagogastric cancer. Cancer Epidemiol. 2023;82:102310.
Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer 2009 101:2. 2009;101:S80–6.
Hamilton W, Sharp DJ, Peters TJ, Round AP. Clinical features of prostate cancer before diagnosis: a population-based, case-control study. Br J Gen Pract. 2006;56:756–62.
Rafiq M, Abel GA, Renzi C, Lyratzopoulos G. Inflammatory marker testing in primary care in the year before Hodgkin lymphoma diagnosis: a UK population-based case–control study in patients aged ≤50 years. Br J Gen Pract. 2022;72:e546–55.
Shephard EA, Neal RD, Rose PW, Walter FM, Hamilton WT. Quantifying the risk of Hodgkin lymphoma in symptomatic primary care patients aged ≥40 years: a case–control study using electronic records. Br J Gen Pract. 2015;65:e289.
Hamilton W, Kernick D. Clinical features of primary brain tumours: a case–control study using electronic primary care records. Br J Gen Pract. 2007;57:695.
McDonald L, Carroll R, Harish A, Tanna N, Mehmud F, Alikhan R, et al. Suspected cancer symptoms and blood test results in primary care before a diagnosis of lung cancer: a case-control study. Future Oncol. 2019;15:3755–62.
Koshiaris C, van den Bruel A, Oke JL, Nicholson BD, Shephard E, Braddick M, et al. Early detection of multiple myeloma in primary care using blood tests: a case–control study in primary care. Br J Gen Pract. 2018;68:e586–93.
Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W. The risk of pancreatic cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2012;106:1940–4.
White B, Rafiq M, Gonzalez-Izquierdo A, Hamilton W, Price S, Lyratzopoulos G. Risk of cancer following primary care presentation with fatigue: a population-based cohort study of a quarter of a million patients. Br J Cancer. 2022;126:1627–36.
Herbert A, Rafiq M, Pham TM, Renzi C, Abel GA, Price S, et al. Predictive values for different cancers and inflammatory bowel disease of 6 common abdominal symptoms among more than 1.9 million primary care patients in the UK: a cohort study. PLoS Med. 2021;18:e1003708.
Watson J, Jones HE, Banks J, Whiting P, Salisbury C, Hamilton W. Use of multiple inflammatory marker tests in primary care: using Clinical Practice Research Datalink to evaluate accuracy. Br J Gen Pract. 2019;69:e462–9.
Hopkins R, Bailey SER, Hamilton WT, Shephard EA. Microcytosis as a risk marker of cancer in primary care: a cohort study using electronic patient records. Br J Gen Pract. 2020;70:e457–62.
Nicholson BD, Oke JL, Aveyard P, Hamilton WT, Hobbs FDR. Individual inflammatory marker abnormalities or inflammatory marker scores to identify primary care patients with unexpected weight loss for cancer investigation? Br J Cancer. 2021;124:1540–2.
Nicholson BD, Hamilton W, Koshiaris C, Oke JL, Hobbs FDR, Aveyard P. The association between unexpected weight loss and cancer diagnosis in primary care: a matched cohort analysis of 65,000 presentations. Br J Cancer. 2020;122:1848.
Barrett J, Hamilton W. Pathways to the diagnosis of lung cancer in the UK: a cohort study. BMC Fam Pr. 2008;9:31.
Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer. 2009;101:S5.
Walter F, Webster A, Scott S, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17:110–8.
Danckert B, Christensen NL, Falborg AZ, Frederiksen H, Lyratzopoulos G, McPhail S, et al. Assessing how routes to diagnosis vary by the age of patients with cancer: a nationwide register-based cohort study in Denmark. BMC Cancer. 2022;22:906.
Abel GA, Mendonca SC, McPhail S, Zhou Y, Elliss-Brookes L, Lyratzopoulos G. Emergency diagnosis of cancer and previous general practice consultations: insights from linked patient survey data. Br J Gen Pr. 2017;67:e377–87.
Herbert A, Abel GA, Winters S, McPhail S, Elliss-Brookes L, Lyratzopoulos G. Cancer diagnoses after emergency GP referral or A&E attendance in England: determinants and time trends in Routes to Diagnosis data, 2006-2015. Br J Gen Pr. 2019;69:E724–30.
Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106:1262–7.
van Erp NF, Helsper CW, Slottje P, Brandenbarg D, Büchner FL, van Asselt KM, et al. Time to diagnosis of symptomatic gastric and oesophageal cancer in the Netherlands: where is the room for improvement? U Eur Gastroenterol J. 2020;8:607.
Koo MM, Lyratzopoulos G, Herbert A, Abel GA, Taylor RM, Barber JA, et al. Association of self-reported presenting symptoms with timeliness of help-seeking among adolescents and young adults with cancer in the BRIGHTLIGHT study. JAMA Netw Open. 2020;3:e2015437.
Soomers VLMN, van der Graaf WTA, Zaidi S, Kaal SEJ, Hayes AJ, Schreuder BHWB, et al. The route to diagnosis of sarcoma patients: results from an interview study in the Netherlands and the United Kingdom. PLoS One. 2020;15:e0243439.
Wiering B, Lyratzopoulos G, Hamilton W, Campbell J, Abel G. Concordance with urgent referral guidelines in patients presenting with any of six ‘alarm’ features of possible cancer: a retrospective cohort study using linked primary care records. BMJ Qual Saf. 2021;31:579–89. bmjqs-2021-013425.
Nadpara P, Madhavan SS, Tworek C. Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: a population-based study. Cancer Epidemiol. 2015;39:1136–44.
Elliss-Brookes L, McPhail S, Ives A, Greenslade M, Shelton J, Hiom S, et al. Routes to diagnosis for cancer – determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107:1220.
Lung Clinical Expert Group. National Optimal Lung Cancer Pathway. 2017. https://www.cancerresearchuk.org/sites/default/files/national_optimal_lung_pathway_aug_2017.pdf. Accessed 8 Feb 2023.
Din NU, Ukoumunne OC, Rubin G, Hamilton W, Carter B, Stapley S, et al. Age and gender variations in cancer diagnostic intervals in 15 cancers: analysis of data from the UK clinical practice research datalink. PLoS One. 2015;10:e0127717.
Neal RD, Din NU, Hamilton W, Ukoumunne OC, Carter B, Stapley S, et al. Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer. 2014;110:584–92.
Price S, Spencer A, Zhang X, Ball S, Lyratzopoulos G, Mujica-Mota R, et al. Trends in time to cancer diagnosis around the period of changing national guidance on referral of symptomatic patients: a serial cross-sectional study using UK electronic healthcare records from 2006-17. Cancer Epidemiol. 2020;69:101805.
Tørring ML, Falborg AZ, Jensen H, Neal RD, Weller D, Reguilon I, et al. Advanced-stage cancer and time to diagnosis: an International Cancer Benchmarking Partnership (ICBP) cross-sectional study. Eur J Cancer Care. 2019;28:e13100.
Falborg AZ, Vedsted P, Menon U, Weller D, Neal RD, Reguilon I, et al. Agreement between questionnaires and registry data on routes to diagnosis and milestone dates of the cancer diagnostic pathway. Cancer Epidemiol. 2020;65:101690.
Lyratzopoulos G, Vedsted P, Singh H. Understanding missed opportunities for more timely diagnosis of cancer in symptomatic patients after presentation. Br J Cancer. 2015;112:S84–91.
Jensen H, Nissen A, Vedsted P. Quality deviations in cancer diagnosis: prevalence and time to diagnosis in general practice. Br J Gen Pr. 2014;64:e92–8.
Swann R, Lyratzopoulos G, Rubin G, Pickworth E, McPhail S. The frequency, nature and impact of GP-assessed avoidable delays in a population-based cohort of cancer patients. Cancer Epidemiol. 2020;64:101617.
Cheraghi-Sohi S, Holland F, Singh H, Danczak A, Esmail A, Morris RL, et al. Incidence, origins and avoidable harm of missed opportunities in diagnosis: longitudinal patient record review in 21 English general practices. BMJ Qual Saf. 2021;30:977–85.
Singh H, Giardina TD, Forjuoh SN, Reis MD, Kosmach S, Khan MM, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21:93–100.
Murphy DR, Meyer AND, Sittig DF, Meeks DW, Thomas EJ, Singh H. Application of electronic trigger tools to identify targets for improving diagnostic safety. BMJ Qual Saf. 2019;28:151–9.
Singh H, Hirani K, Kadiyala H, Rudomiotov O, Davis T, Khan MM, et al. Characteristics and predictors of missed opportunities in lung cancer diagnosis: an electronic health record-based study. J Clin Oncol. 2010;28:3307–15.
Murphy DR, Laxmisan A, Reis BA, Thomas EJ, Esquivel A, Forjuoh SN, et al. Electronic health record-based triggers to detect potential delays in cancer diagnosis. BMJ Qual Saf. 2014;23:8–16.
Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27:557.
Miller AC, Arakkal AT, Koeneman S, Cavanaugh JE, Gerke AK, Hornick DB, et al. Incidence, duration and risk factors associated with delayed and missed diagnostic opportunities related to tuberculosis: a population-based longitudinal study. BMJ Open. 2021;11:e045605.
Fischhoff B. Hindsight not equal to foresight: the effect of outcome knowledge on judgment under uncertainty. Qual Saf Health Care. 2003;12:304–11. 1975
Zhou Y, Mendonca SC, Abel GA, Hamilton W, Walter FM, Johnson S, et al. Variation in ‘fast-track’ referrals for suspected cancer by patient characteristic and cancer diagnosis: evidence from 670 000 patients with cancers of 35 different sites. Br J Cancer. 2018;118:24.
Nygaard C, Jensen H, Christensen J, Vedsted P. Health care use before a diagnosis of primary intracranial tumor: a Danish nationwide register study. Clin Epidemiol. 2018;10:809.
Renzi C, Lyratzopoulos G, Card T, Chu TPC, Macleod U, Rachet B. Do colorectal cancer patients diagnosed as an emergency differ from non-emergency patients in their consultation patterns and symptoms? A longitudinal data-linkage study in England. Br J Cancer. 2016;115:866–75.
Renzi C, Lyratzopoulos G, Hamilton W, Maringe C, Rachet B. Contrasting effects of comorbidities on emergency colon cancer diagnosis: a longitudinal data-linkage study in England. BMC Health Serv Res. 2019;19:311.
Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92–107.
Pham TM, Gomez-Cano M, Salika T, Jardel D, Abel GA, Lyratzopoulos G. Diagnostic route is associated with care satisfaction independently of tumour stage: evidence from linked English Cancer Patient Experience Survey and cancer registration data. Cancer Epidemiol. 2019;61:70–78.
Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316:1818–9.
Tørring ML, Frydenberg M, Hamilton W, Hansen RP, Lautrup MD, Vedsted P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol. 2012;65:669–78.
Crawford SC, Davis JA, Siddiqui NA, De Caestecker L, Gillis CR, Hole D. The waiting time paradox: population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. BMJ Br Med J. 2002;325:196.
Emery J, Brown G, Macrae F, Bell C, Tse J, Skinner I, et al. Optimal maximum time from referral to diagnosis and treatment. 2017. https://wiki.cancer.org.au/australia/Clinical_question:Diagnosis_interval_in_colorectal_cancer. Accessed 29 Jun 2023.
Forrest LF, Adams J, Rubin G, White M. The role of receipt and timeliness of treatment in socioeconomic inequalities in lung cancer survival: population-based, data-linkage study. Thorax. 2015;70:138–45.
Hofmann B. Getting personal on overdiagnosis: On defining overdiagnosis from the perspective of the individual person. J Eval Clin Pr. 2018;24:983–7.
Carter JL, Coletti RJ, Harris RP. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ. 2015;350:g7773.
White B, Renzi C, Rafiq M, Abel GA, Jensen H, Lyratzopoulos G. Does changing healthcare use signal opportunities for earlier detection of cancer? A review of studies using information from electronic patient records. Cancer Epidemiol. 2022;76:102072.
Lawrenson R, Logie J, Marks C. Risk of colorectal cancer in general practice patients presenting with rectal bleeding, change in bowel habit or anaemia. Eur J Cancer Care (Engl). 2006;15:267–71.
Barrett J, Jiwa M, Rose P, Hamilton W. Pathways to the diagnosis of colorectal cancer: an observational study in three UK cities. Fam Pract. 2006;23:15–9.
Singh H, Daci K, Petersen LA, Collins C, Petersen NJ, Shethia A, et al. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am J Gastroenterol. 2009;104:2543–54.
Funding
EW acknowledges the receipt of a studentship award from the Health Data Research UK-The Alan Turing Institute Wellcome PhD Programme in Health Data Science (Grant Ref: 218529/Z/19/Z). BW, MEB, and GL were supported by a Cancer Research UK (C18081/A18180) Advanced Clinician Scientist Fellowship (to GL). MEB is supported by a Cancer Research UK International Alliance for Cancer Early Detection (ACED) Pathway Award (EDDAPA-2022/100002). CR acknowledges funding from Cancer Research UK — Early Detection and Diagnosis Committee (EDDCPJT/100018). The study aligns to (but was not directly supported by) the RREDD-EHR project supported by the International Alliance for Cancer Early Detection a partnership between Cancer Research UK (C18081/A31373), Canary Center at Stanford University, the University of Cambridge, OHSU Knight Cancer Institute, University College London, and the University of Manchester. SD is funded from: (a) Health Data Research UK, which receives its funding from HDR UK Ltd (HDR-9006) funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation (BHF) and the Wellcome Trust. (b) the BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking under Grant agreement No 116074., (c) the NIHR UCLH Biomedical Research Centre, (d) an Alan Turing Fellowship (EP/N510129/1), (e) the Longitudinal Health and Wellbeing COVID-19 National Core Study, which was established by the UK Chief Scientific Officer in October 2020 and funded by UK Research and Innovation (Grant references MC_PC_20030 and MC_PC_20059), by the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (Grant reference MC_PC_20058), and by the CONVALESCENCE study of long COVID, which is funded by NIHR/UKRI, (f) The British Heart Foundation Data Science Centre (Grant No SP/19/3/34678, awarded to Health Data Research (HDR) UK).
Author information
Authors and Affiliations
Contributions
EW conceived the article. All authors contributed to drafting and revising the article.
Corresponding author
Ethics declarations
Competing interests
MEB receives personal fees from GRAIL Inc., for Independent Data Monitoring Committee (IDMC) membership unrelated to this study. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Whitfield, E., White, B., Denaxas, S. et al. A taxonomy of early diagnosis research to guide study design and funding prioritisation. Br J Cancer 129, 1527–1534 (2023). https://doi.org/10.1038/s41416-023-02450-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02450-4