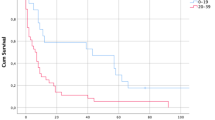
Abstract
Peritoneal metastases from various abdominal cancer types are common and carry poor prognosis. The presence of peritoneal disease upstages cancer diagnosis and alters disease trajectory and treatment pathway in many cancer types. Therefore, accurate and timely detection of peritoneal disease is crucial. The current practice of diagnostic laparoscopy and peritoneal lavage cytology (PLC) in detecting peritoneal disease has variable sensitivity. The significant proportion of peritoneal recurrence seen during follow-up in patients where initial PLC was negative indicates the ongoing need for a better diagnostic tool for detecting clinically occult peritoneal disease, especially peritoneal micro-metastases. Advancement in liquid biopsy has allowed the development and use of peritoneal tumour DNA (ptDNA) as a cancer-specific biomarker within the peritoneum, and the presence of ptDNA may be a surrogate marker for early peritoneal metastases. A growing body of literature on ptDNA in different cancer types portends promising results. Here, we conduct a systematic review to evaluate the prognostic impact of ptDNA in various cancer types and discuss its potential future clinical applications, with a focus on gastrointestinal and gynaecological malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kus T, Kose F, Aktas G, Arslan UY, Sedef AM, Cinkir HY, et al. Prediction of peritoneal recurrence in patients with gastric cancer: a multicenter study. J Gastrointest Cancer. 2021;52:634–42.
Suenaga M, Fujii T, Yamada S, Hayashi M, Shinjo K, Takami H, et al. Peritoneal lavage tumor DNA as a novel biomarker for predicting peritoneal recurrence in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2021;28:2277–86.
López-Rojo I, Olmedillas-López S, Villarejo Campos P, Domínguez Prieto V, Barambio Buendía J, Cortés, et al. Liquid biopsy in peritoneal fluid and plasma as a prognostic factor in advanced colorectal and appendiceal tumors after complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ther Adv Med Oncol. 2020;12:1758835920981351.
Swisher EM, Wollan M, Mahtani SM, Willner JB, Garcia R, Goff BA, et al. Tumor-specific p53 sequences in blood and peritoneal fluid of women with epithelial ovarian cancer. Am J Obstet Gynecol. 2005;193:662–7.
Cho JH, Kim SS. Peritoneal carcinomatosis and its mimics: review of CT findings for differential diagnosis. J Belg Soc Radiol. 2020;104:8.
Zeppernick F, Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch Gynecol Obstet. 2014;290:839–42.
Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012;15:S27–37.
Nomoto S, Nakao A, Kasai Y, Inoue S, Harada A, Nonami T, et al. Peritoneal washing cytology combined with immunocytochemical staining and detecting mutant K-ras in pancreatic cancer: comparison of the sensitivity and availability of various methods. Pancreas. 1997;14:126–32.
Zuna RE, Behrens A. Peritoneal washing cytology in gynecologic cancers: long-term follow-up of 355 patients. J Natl Cancer Inst. 1996;88:980–7.
Razenberg LG, van Gestel YR, Creemers GJ, Verwaal VJ, Lemmens VE, de Hingh IH. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur J Surg Oncol. 2015;41:466–71.
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57.
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384:1191–203.
Nakauchi M, Vos EL, Carr RA, Barbetta A, Tang LH, Gonen M, et al. Distinct differences in gastroesophageal junction and gastric adenocarcinoma in 2194 patients: in memory of Rebecca A. Carr, February 24, 1988-January 19, 2021. Ann Surg. 2023;277:629–36.
Siravegna G, Mussolin B, Venesio T, Marsoni S, Seoane J, Dive C, et al. How liquid biopsies can change clinical practice in oncology. Ann Oncol. 2019;30:1580–90.
Tivey A, Church M, Rothwell D, Dive C, Cook N. Circulating tumour DNA—looking beyond the blood. Nat Rev Clin Oncol. 2022;19:600–12.
Lam RCT, Johnson D, Lam G, Li MLY, Wong JWL, Lam WKJ, et al. Clinical applications of circulating tumor-derived DNA in the management of gastrointestinal cancers—current evidence and future directions. Front Oncol. 2022;12:970242.
Lemoine L, Sugarbaker P, Van der Speeten K. Pathophysiology of colorectal peritoneal carcinomatosis: role of the peritoneum. World J Gastroenterol. 2016;22:7692–707.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6.
Zhou S, Xu B, Qi L, Zhu D, Liu B, Wei J. Next-generation sequencing reveals mutational accordance between cell-free DNA from plasma, malignant pleural effusion and ascites and directs targeted therapy in a gastric cancer patient. Cancer Biol Ther. 2019;20:15–20.
Ju HY, Ho JY, Kang J, Hur SY, Kim S, Choi YJ, et al. Whole-exome sequencing reveals clinical potential of circulating tumor DNA from peritoneal fluid and plasma in endometrial cancer. Cancers. 2022;14:2506.
Mayo-de-Las-Casas C, Velasco A, Sanchez D, Martínez-Bueno A, Garzón-Ibáñez M, Gatius S, et al. Detection of somatic mutations in peritoneal lavages and plasma of endometrial cancer patients: a proof-of-concept study. Int J Cancer. 2020;147:277–84.
Werner B, Yuwono N, Duggan J, Liu D, David C, Srirangan S, et al. Cell-free DNA is abundant in ascites and represents a liquid biopsy of ovarian cancer. Gynecol Oncol. 2021;162:720–7.
Han MR, Lee SH, Park JY, Hong H, Ho JY, Hur SY, et al. Clinical implications of circulating tumor DNA from ascites and serial plasma in ovarian cancer. Cancer Res. Treat. 2020;52:779–788.
Barquín M, Maximiano C, Pérez-Barrios C, Sanchez-Herrero E, Soriano M, Colmena M, et al. Peritoneal washing is an adequate source for somatic BRCA1/2 mutation testing in ovarian malignancies. Pathol Res Pr. 2019;215:392–4.
Krimmel JD, Schmitt MW, Harrell MI, Agnew KJ, Kennedy SR, Emond MJ, et al. Ultra-deep sequencing detects ovarian cancer cells in peritoneal fluid and reveals somatic TP53 mutations in noncancerous tissues. Proc Natl Acad Sci USA. 2016;113:6005–10.
Parrella P, Zangen R, Sidransky D, Nicol T. Molecular analysis of peritoneal fluid in ovarian cancer patients. Mod Pathol. 2003;16:636–40.
Dokianakis DN, Varras MN, Papaefthimiou M, Apostolopoulou J, Simiakaki H, Diakomanolis E, et al. Ras gene activation in malignant cells of human ovarian carcinoma peritoneal fluids. Clin Exp Metastasis. 1999;17:293–7.
Nozaki T, Sakamoto I, Kagami K, Amemiya K, Hirotsu Y, Mochizuki H, et al. Molecular analysis of ascitic fluid cytology reflects genetic changes of malignancies of the ovary equivalent to surgically resected specimens. Cancer Cytopathol. 2022;130:640–9.
Hickey KP, Boyle KP, Jepps HM, Andrew AC, Buxton EJ, Burns PA. Molecular detection of tumour DNA in serum and peritoneal fluid from ovarian cancer patients. Br J Cancer. 1999;80:1803–8.
Ibanez de Caceres I, Battagli C, Esteller M, Herman JG, Dulaimi E, Edelson MI, et al. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004;64:6476–81.
van’t Erve I, Rovers KP, Constantinides A, Bolhuis K, Wassenaar ECE, Lurvink RJ, et al. Detection of tumor-derived cell-free DNA from colorectal cancer peritoneal metastases in plasma and peritoneal fluid. J Pathol Clin Res. 2021;7:203–8.
Kamiyama H, Noda H, Takata O, Md YK, Konishi F. Promoter hypermethylation of tumor-related genes in peritoneal lavage and the prognosis of patients with colorectal cancer. J Surg Oncol. 2009;100:69–74.
Leick KM, Kazarian AG, Rajput M, Tomanek-Chalkley A, Miller A, Shrader HR, et al. Peritoneal cell-free tumor DNA as biomarker for peritoneal surface malignancies. Ann Surg Oncol. 2020;27:5065–71.
Yuan Z, Chen W, Liu D, Qin Q, Grady WM, Fichera A, et al. Peritoneal cell-free DNA as a sensitive biomarker for detection of peritoneal metastasis in colorectal cancer: a prospective diagnostic study: a prospective diagnostic study. Clin Epigenet. 2023;15:65.
Chiba K, Hata T, Mizuma M, Masuda K, Aoki S, Takadate T, et al. Impact of tumor-derived DNA testing in peritoneal lavage of pancreatic cancer patients with and without occult intra-abdominal metastases. Ann Surg Oncol. 2022;29:2685–97.
Yonkus JA, Alva-Ruiz R, Abdelrahman AM, Leiting JL, Schneider AR, Grotz TE, et al. Molecular peritoneal staging for pancreatic ductal adenocarcinoma using mutant KRAS droplet-digital polymerase chain reaction: results of a prospective clinical trial. J Am Coll Surg. 2021;233:73-+.
Hiraki M, Kitajima Y, Sato S, Nakamura J, Hashiguchi K, Noshiro H, et al. Aberrant gene methylation in the peritoneal fluid is a risk factor predicting peritoneal recurrence in gastric cancer. World J Gastroenterol. 2010;16:330–8.
Hiraki M, Kitajima Y, Koga Y, Tanaka T, Nakamura J, Hashiguchi K, et al. Aberrant gene methylation is a biomarker for the detection of cancer cells in peritoneal wash samples from advanced gastric cancer patients. Ann Surg Oncol. 2011;18:3013–9.
Yu QM, Wang XB, Luo J, Wang S, Fang XH, Yu JL, et al. CDH1 methylation in preoperative peritoneal washes is an independent prognostic factor for gastric cancer. J Surg Oncol. 2012;106:765–71.
Ushiku H, Yamashita K, Ema A, Minatani N, Kikuchi M, Kojo K, et al. DNA diagnosis of peritoneal fluid cytology test by CDO1 promoter DNA hypermethylation in gastric cancer. Gastric Cancer. 2017;20:784–92.
Harada H, Soeno T, Nishizawa N, Washio M, Sakuraya M, Ushiku H, et al. Prospective study to validate the clinical utility of DNA diagnosis of peritoneal fluid cytology test in gastric cancer. Cancer Sci. 2021;112:1644–54.
Yukawa N, Yamada T, Aoyama T, Woo T, Ueda K, Mastuda A, et al. Tumor DNA in Peritoneal lavage as a novel biomarker for predicting peritoneal recurrence in patients with gastric cancer. Anticancer Res. 2023;43:2069–76.
Yamashita K, Kuba T, Shinoda H, Takahashi E, Okayasu I. Detection of K-ras point mutations in the supernatants of peritoneal and pleural effusions for diagnosis complementary to cytologic examination. Am J Clin Pathol. 1998;109:704–11.
Jayne D. Molecular biology of peritoneal carcinomatosis. Cancer Treat Res. 2007;134:21–33.
Yonemura Y, Ishibashi H, Mizumoto A, Tukiyama G, Liu Y, Wakama S, et al. The development of peritoneal metastasis from gastric cancer and rationale of treatment according to the mechanism. J Clin Med. 2022;11:458.
Virgilio E, Giarnieri E, Giovagnoli MR, Montagnini M, Proietti A, D’Urso R, et al. Gastric cancer cells in peritoneal lavage fluid: a systematic review comparing cytological with molecular detection for diagnosis of peritoneal metastases and prediction of peritoneal recurrences. Anticancer Res. 2018;38:1255–62.
Wu HT, Ji HN, Yang WH, Zhang M, Guo YF, Li BK, et al. Liquid biopsy using ascitic fluid and pleural effusion supernatants for genomic profiling in gastrointestinal and lung cancers. BMC Cancer. 2022;22:1020.
Husain H, Nykin D, Bui N, Quan D, Gomez G, Woodward B, et al. Cell-Free DNA from ascites and pleural effusions: molecular insights into genomic aberrations and disease biology. Mol Cancer Ther. 2017;16:948–55.
Nelson AC, Boone J, Cartwright D, Thyagarajan B, Kincaid R, Lambert AP, et al. Optimal detection of clinically relevant mutations in colorectal carcinoma: sample pooling overcomes intra-tumoral heterogeneity. Mod Pathol. 2018;31:343–9.
Jung M, Putzer S, Gevensleben H, Meller S, Kristiansen G, Dietrich D. Diagnostic and prognostic value of SHOX2 and SEPT9 DNA methylation and cytology in benign, paramalignant, and malignant ascites. Clinical Epigenet. 2016;8:1–13.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 15th Edition. Oxford, UK: Kanehara-Shuppan; 2017.
Chang HW, Ali SZ, Cho SKR, Kurman RJ, Shih IM. Detection of allelic imbalance in ascitic supernatant by digital single nucleotide polymorphism analysis. Clin Cancer Res. 2002;8:2580–5.
Pu XL, Li ZY, Wang XY, Jiang H. Ascites and serial plasma circulating tumor DNA for predicting the effectiveness of hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis. Front Oncol. 2022;12:791418.
Funding
Dr. David S Liu received research funding from the North Eastern Melbourne Integrated Cancer Service (Service Improvement Grant), Peter MacCallum Cancer Foundation (Discovery Partner Fellowship), Austin Medical Research Foundation (Grant-In-Aid), and the Royal Australasian College of Surgeons (Paul Mackay Bolton Grant for Cancer Research).
Author information
Authors and Affiliations
Contributions
ZA performed the systematic review search, drafted and revised the manuscript; SW performed the systematic review search and revised the manuscript; JT, NT, NC and DL supervised the systematic review search and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Allan, Z., Witts, S., Tie, J. et al. The prognostic impact of peritoneal tumour DNA in gastrointestinal and gynaecological malignancies: a systematic review. Br J Cancer 129, 1717–1726 (2023). https://doi.org/10.1038/s41416-023-02424-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02424-6