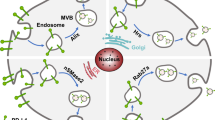
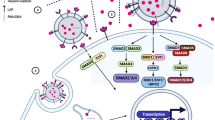
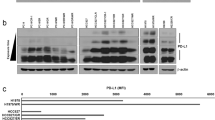
Abstract
Background
The PD-L1 on tumor cell-derived small extracellular vesicles (sEVs) can suppress the proliferation and cytokine production of T cells. However, PD-L1 can also be expressed by non-tumor cells. The present study is designed to test whether immunocytes release immunosuppressive PD-L1-positive sEVs.
Methods
sEVs were isolated from different clinical samples of head and neck squamous cell carcinoma (HNSCC) patients, the level and cellular origins of PD-L1-positive sEVs were assessed. Co-expression of CD80 on PD-L1-positive sEVs was examined to evaluate the immunosuppressive and tumor-promotive effects.
Results
PD-L1-positive sEVs in HNSCC patients had various cellular origins, including tumor cell, T cell, B cell, dendritic cell and monocyte/macrophage. However, PD-L1-positive sEVs derived from immune cells did not exert immunosuppressive functions due to the co-expression of CD80. It was verified that co-expression of CD80 disrupted the binding of sEV PD-L1 to its receptor PD-1 on T cells and attenuated the immunosuppression mediated by sEV PD-L1 both in vitro and in vivo.
Conclusion
The study suggests that PD-L1-positive sEVs have the cellular origin and functional heterogeneity. Co-expression of CD80 could restrict the immunosuppressive effect of sEV PD-L1. A greater understanding of PD-L1-positive sEV subsets is required to further improve their clinical application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Dong H, Strome S, Salomao D, Tamura H, Hirano F, Flies D, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800.
Poggio M, Hu T, Pai C, Chu B, Belair C, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–27.e413.
Chen G, Huang A, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–6.
Robbins P, Morelli A. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208.
Cordonnier M, Nardin C, Chanteloup G, Derangere V, Algros M, Arnould L, et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9:1710899.
Serratì S, Guida M, Di Fonte R, De Summa S, Strippoli S, Iacobazzi R, et al. Circulating extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma. Mol Cancer. 2022;21:20.
Timaner M, Kotsofruk R, Raviv Z, Magidey K, Shechter D, Kan T, et al. Microparticles from tumors exposed to radiation promote immune evasion in part by PD-L1. Oncogene. 2020;39:187–203.
Li M, Soder R, Abhyankar S, Abdelhakim H, Braun M, Trinidad C, et al. WJMSC-derived small extracellular vesicle enhance T cell suppression through PD-L1. J Extracell Vesicles. 2021;10:e12067.
Keir M, Butte M, Freeman G, Sharpe A. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Herbst R, Soria J, Kowanetz M, Fine G, Hamid O, Gordon M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7.
Theodoraki M, Hoffmann T, Whiteside T. Separation of plasma-derived exosomes into CD3 and CD3 fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin Exp Immunol. 2018;192:271–83.
Sugiura D, Maruhashi T, Okazaki I, Shimizu K, Maeda T, Takemoto T, et al. cisRestriction of PD-1 function by -PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364:558–66.
Zhao Y, Lee C, Lin C, Gassen R, Xu X, Huang Z, et al. PD-L1:CD80 Cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity. 2019;51:1059–73.e1059.
Stein J, Lipson E, Cottrell T, Forde P, Anders R, Cimino-Mathews A, et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade. Clin Cancer Res. 2020;26:545–51.
Tian Y, Ma L, Gong M, Su G, Zhu S, Zhang W, et al. Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano. 2018;12:671–80.
Cohen E. Role of epidermal growth factor receptor pathway-targeted therapy in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2659–65.
Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3–20.
Zhao Y, Harrison D, Song Y, Ji J, Huang J, Hui E. Antigen-presenting cell-intrinsic PD-1 neutralizes PD-L1 in cis to attenuate PD-1 signaling in T cells. Cell Rep. 2018;24:379–90.e376.
Chaudhri A, Xiao Y, Klee A, Wang X, Zhu B, Freeman G. In CisPD-L1 binds to B7-1 only on the same cell surface. Cancer Immunol Res. 2018;6:921–9.
Haile S, Bosch J, Agu N, Zeender A, Somasundaram P, Srivastava M, et al. Tumor cell programmed death ligand 1-mediated T cell suppression is overcome by coexpression of CD80. J Immunol. 2011;186:6822–9.
Zhang W, Zhong W, Wang B, Yang J, Yang J, Yu Z, et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev Cell. 2022;57:329–43.e327.
Chen L, Flies D. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42.
Sharpe A, Pauken K. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67.
Sugiura D, Okazaki I, Maeda T, Maruhashi T, Shimizu K, Arakaki R, et al. PD-1 agonism by anti-CD80 inhibits T cell activation and alleviates autoimmunity. Nat Immunol. 2022;23:399–410.
Wang S, Shi Y. Exosomes derived from immune cells: the new role of tumor immune microenvironment and tumor therapy. Int J Nanomedicine. 2022;17:6527–50.
Choi S, Cho H, Yea K, Baek M. Immune cell-derived small extracellular vesicles in cancer treatment. BMB Rep. 2022;55:48–56.
Qiu Y, Yang Y, Yang R, Liu C, Hsu J, Jiang Z, et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene. 2021;40:4992–5001.
Wang X, Shen H, He Q, Tian W, Xia A, Lu X. Exosomes derived from exhausted CD8 + T cells impaired the anticancer function of normal CD8 + T cells. J Med Genet. 2019;56:29–31.
Funding
This work was supported by the National Key R&D Program of China (2019YFA0210500), National Natural Science Foundation of China (82341023, 81922038, 81801842), Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (TFJC2018005), The Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZLCX20212300), The Fundamental Research Funds for the Central Universities (2042022dx0003).
Author information
Authors and Affiliations
Contributions
GC conceived and designed the study. JYL, ZLZ, QYF and JBL performed the experiments. JYL, ZLY, MW and BL analyzed and interpreted the data. GC, JYL and ZLY wrote and edited the manuscript. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of School and Hospital of Stomatology, Wuhan University (IRB no.2022.B05) and Zhongnan Hospital of Wuhan University (IRB no. 2021059 K). Participants gave informed consent to participate in the study before taking part. The permission for animals was approved by the Institutional Research Ethics Committee of School and Hospital of Stomatology, Wuhan University (IRB no. S07921060C).
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, JY., Yu, ZL., Fu, QY. et al. Immunosuppressive effect of small extracellular vesicle PD-L1 is restricted by co-expression of CD80. Br J Cancer 129, 925–934 (2023). https://doi.org/10.1038/s41416-023-02369-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02369-w