Abstract
Background
The association between excess weight and colorectal cancer (CRC) risk may have been underestimated due to potential weight loss during pre-clinical sojourn time of CRC. We aimed to investigate this association and the corresponding population attributable fraction (PAF), accounting for prediagnostic weight loss.
Methods
Data from the UK Biobank prospective cohort were used. Multivariable adjusted hazard ratios (HR) and their 95% confidence intervals (CI) for various periods of follow-up and the corresponding PAF of excess weight were calculated.
Results
During a median of 10.0 years of follow-up, of 453,049 participants, 4794 developed CRC. The excess weight–CRC association became substantially stronger with including increasing lengths of follow-up in the analyses and further excluding the initial years of follow-up. HRs (95% CIs) for overweight and obesity were 1.06 (0.97–1.16) and 1.14 (1.03–1.26) after 7 years of follow-up, 1.13 (1.05–1.21) and 1.23 (1.14–1.33) when including complete follow-up length, and 1.26 (1.12–1.43) and 1.42 (1.24–1.63) when excluding the initial 7 years of follow-up. The corresponding PAFs of excess weight were estimated as 6.8%, 11.3%, and 19.0%, respectively.
Conclusions
Comprehensive consideration of the potential effect of prediagnostic weight loss discloses a much stronger impact of excess body weight on CRC risk than previously assumed.
Similar content being viewed by others
Background
Excess body weight, often represented as increased body mass index (BMI), is associated with higher risk of different cancers including colorectal cancer (CRC) [1, 2]. The incidence of CRC is growing especially in younger populations and formerly low-risk countries in which changes in life-style factors, including diet, e.g., higher meat consumption, sedentary life-style, and less physical activity resulting in excess body weight and unfavourable body fat distribution, play an important role [3]. The prevalence of overweight (BMI ≥ 25 to 30 kg/m2) and obesity (BMI ≥ 30 kg/m2) has increased rapidly over the last few decades [4].
The population attributable fraction (PAF) of overweight and obesity for CRC has been estimated between 5 and 11% in different populations [5,6,7,8,9]. Furthermore, recent systematic reviews and meta-analyses have reported approximately 18% and 30% increase in CRC risk for overweight and obesity, respectively, compared to normal weight [10, 11]. However, there are concerns that the impact of excess body weight may have been underestimated in epidemiological studies due to prediagnostic weight loss [12], which is common among CRC patients [13]. This particularly applies to case-control studies, in which BMI is commonly ascertained close to the time of diagnosis among cases. Nonetheless, prediagnostic weight loss may also affect risk estimates from cohort studies, where BMI is ascertained at baseline prior to diagnosis of CRC during follow-up. The mean sojourn time (average duration of pre-clinical phase) of CRC has been estimated 3 to 6 years [14,15,16], and a relevant proportion of cancers diagnosed in the early years of follow-up may have existed and led to weight loss already at recruitment.
We aimed to assess the potential role of prediagnostic weight loss during pre-clinical sojourn time on estimates of the excess weight–CRC risk association in a large prospective cohort with about half a million study participants, the UK Biobank.
Methods
Study design and study participants
The UK Biobank is a prospective cohort that has collected extensive data on socio-demographic, life-style, and health-related factors through a self-completed touch-screen questionnaire and a computer assisted interview from about half a million study participants across UK aged 40–69 years when recruited (2006–2010). The participants have also undergone physical and functional measurements and cancer, death, and primary care data are available through linkage to electronic health records as previously described elsewhere [17]. The UK Biobank has ethical approval from the North West Multi-centre Research Ethics Committee (MREC) as a Research Tissue Bank (RTB), approval renewed in 2021 (21/NW/0157) and all participants provided electronic signed informed consent. This analysis was restricted to men and women with no previous cancer diagnosis and complete information on BMI at recruitment.
Assessment of exposure
BMI values were determined by dividing weight in kilograms by the square of height in metres. Weight was measured using the Tanita BC-418 MA body composition analyser and standing height using a Seca 202 height measure as part of the initial assessment visit [18]. BMI (kg/m2) was classified according to the World Health Organization (WHO) categories: <18.5 (underweight), ≥18.5 to <25 (normal weight), ≥25 to 30 (overweight), ≥30 to <35 (obesity class I), ≥35 to <40 (obesity class II), and ≥40 (obesity class III) [19].
Assessment of outcome
The coded data on cancer incidence (10th revision of the International Statistical Classification of Diseases (ICD-10)) are provided by the UK Biobank through linkage to national cancer registries. This analysis is based on cancer incidence follow-up to 31 July 2019 for England and Wales and 31 October 2015 for Scotland. CRC cases were defined as incident malignant neoplasms of the colon (C18), rectosigmoid junction (C19) and rectum (C20).
Assessment of covariates
Data regarding socio-demographics, life-style, health, and medical history and medication use were collected at initial assessment visits. Age, ethnicity (white, Asian, black, mixed, and other), socio-economic status (Townsend deprivation index), educational qualifications (higher academic/professional, lower academic/vocational, or none), smoking status (never, former, current) alcohol consumption (never, at special occasions only, one to three times a month, once or twice a week, three or four times a week, daily, or almost daily), and level of physical activity (low, moderate, and high) according to international physical activity questionnaire (IPAQ) [20] were ascertained. Frequency and type of food intake collected via touch-screen questionnaire was used to determine fruit (pieces/day), vegetable (tablespoons/day), and red and processed meat intake, categorised into never, less than once a week, once a week, and two or more times a week.
Family history of CRC, history of bowel cancer screening (faecal occult blood test, colonoscopy/sigmoidoscopy), and regular use of non-steroidal anti-inflammatory drugs (NSAIDs) or aspirin were also identified.
Statistical analysis
The baseline characteristics of the cohort are displayed with descriptive statistics. Median (interquartile range (IQR)) of BMI is reported by levels of covariates and compared by Kruskal–Wallis test.
Multivariable Cox proportional hazards models were used to evaluate the association between BMI and the risk of CRC. Follow-up time was defined as the time from cohort entry to the first CRC diagnosis, date of death, date lost to follow-up, or end of follow-up (31 July 2019 for England and Wales and 31 October 2015 for Scotland). Two models were fitted; the first model was adjusted for age (continuous) and sex and the second model was additionally adjusted for ethnicity, socio-economic deprivation (continuous), educational qualification, smoking status, alcohol consumption, physical activity, fruit (continuous), vegetable (continuous), red meat and processed meat consumption, family history of CRC, history of bowel cancer screening, and regular use of NSAIDs or aspirin. Deviations from the proportionality assumption were examined by Schoenfeld residuals plots for each covariate and no deviations were found. Missing covariate values were imputed using PROC MI and the analyses were performed combining the analyses of five imputed datasets using PROC MIANALYZE. The percentage of missing values was 20% for physical activity, and less than 2% for other covariates.
Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated to quantify the risk of CRC per each category of BMI compared to the normal weight as the reference group. The initial analysis used all of the above-mentioned WHO categories. In further analyses, combined categories <25 (normal weight), ≥25 to <30 (overweight), and ≥30 kg/m2 (obesity) were used due the small numbers of participants with BMI < 18.5 and >35 kg/m2.
We first carried out a standard cohort analysis using the full follow-up time available at the time of the analysis. Next, we repeated the analyses, starting with including only the first year of follow-up and then gradually increasing the maximum follow-up time (i.e., the censoring time) in steps of 1 year up to the maximum possible follow-up time of 13 years. The rationale was to gain more insight into the association between BMI and CRC risk in the early years of follow-up, when a substantial proportion of newly diagnosed CRC cases would be expected to have occurred among participants who already had pre-clinical CRC at the time of recruitment, and to see how length of follow-up might affect the derived overall estimates of the BMI-CRC association.
Subsequently, in order to progressively reduce potential bias from prediagnostic weight loss, we first excluded the first year of the follow-up time, which corresponds to a late entry analysis, and then extended this time to 2, 3, 4, 5, 6, 7, and finally first 8 years of follow-up. This was accomplished by excluding participants with follow-up time ≤1, ≤2, ≤3, ≤4, ≤5, ≤6, ≤7, and ≤8 years, respectively, and reducing follow-up time by 1, 2, 3, 4, 5, 6, 7, and 8 years, respectively, among the remaining participants.
We also calculated population attributable fractions (PAFs) that estimate the proportion of CRC cases in the study population that are statistically attributable to these conditions, using Levin’s formula [21]. Due to the predominant occurrence of CRC cases among older and male participants, who have higher prevalences of excess weight, we first calculated age- and sex-specific PAFs and derived the overall PAF as a weighted average of the age- and sex-specific PAFs, with weights equal to the age- and sex-specific numbers of CRC cases.
In addition, subgroup analyses by age group, sex, smoking status and history of bowel cancer screening, and subsite-specific analyses for colon cancer (C18.0–C18.9), proximal colon cancer (C18.0 and C18.2–C18.4), distal colon cancer (C18.5–C18.7), and rectal cancer (C19 and C20) were performed. For each subgroup and each subsite, HRs and PAFs were estimated for three types of analyses: (i) including the initial 7 years of follow-up only, (ii) including the entire follow-up, and (iii) excluding the initial 7 years of follow-up. Furthermore, we investigated the potential interaction between BMI (continuous) and each stratification variable by including the corresponding interaction term in the model. Differences regarding site-specific associations were evaluated by heterogeneity test for colon vs rectal cancer and proximal vs distal colon cancer. Both evaluations were conducted for the complete follow-up time.
All analyses were performed using SAS software version 9.4. Associations with two-sided p-values <0.05 were considered statistically significant.
Results
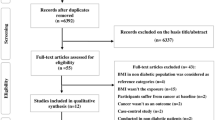
Of 502,422 participants, 8 withdrew consent, 46,530 had cancer at or prior to recruitment and 2835 had missing values for BMI and were excluded from the analyses (Fig. 1). Therefore, 453,049 participants including 4794 CRC cases with median follow-up time of 10.0 years were included in the analyses.
In the total cohort, the median of BMI was 26.8 kg/m2 (IQR: 24.2–29.9). Baseline characteristics of the study population with regard to their measured BMI at baseline are shown in Table 1. The median BMI for men and women were 27.3 kg/m2 (IQR: 25.0–30.1) and 26.1 kg/m2 (IQR: 23.4–29.7), respectively, and participants who had older age, were male, black, had higher Townsend deprivation index, with no educational qualification, were former smokers, had higher frequency of alcohol consumption, and low physical activity had higher median BMI. Participants who consumed <2 and <3 portions of fruit and vegetables per day, or red or processed meat ≥2 times per week, reported history of bowel cancer screening, and family history of CRC also had higher median BMI.
Table 2 shows the association with the risk of CRC for participants with underweight, overweight and obesity class I, II, and III compared to the normal weight participants, using the complete follow-up years included in the analyses. The results from model 1 and model 2 were very similar and therefore, only the results from the fully adjusted model are presented for further analyses. There was a 12% increase in CRC risk for overweight (HR: 1.12, 95% CI: 1.05–1.20), 21% for class I obesity (HR: 1.21, 95% CI: 1.11–1.31), 27% for class II obesity (HR: 1.27, 95% CI: 1.10–1.45), and 34% for class III obesity (HR: 1.34, 95% CI: 1.08–1.65). There was an inverse association between underweight and the risk of CRC but this association was not statistically significant (HR: 0.63, 95% CI: 0.35–1.14).
Table 3 displays the HRs and PAFs for overweight and obesity compared to normal weight estimated with including various follow-up time windows after recruitment in the analysis. Within the initial four years of follow-up, null results with very small, statistically non-significant risk increases were estimated for overweight and obesity. Even after 8 years of follow-up, only a marginally significant 9% increase in risk was estimated for overweight participants. Only after inclusion of 10 or more years of follow-up, quite consistent estimates of significantly increased risk were obtained for both overweight (13–14% risk increase) and obesity (23–24% risk increase). However, even substantially higher estimates were obtained after excluding the initial years of follow-up, which might be affected by pre-clinical weight loss. For example, risk of CRC was estimated to be significantly increased by 16 and 31% beyond the fourth year of follow-up among overweight and participants with obesity, respectively. Risk estimates continued to increase with increasing length of excluded time window after recruitment until the 7th year of follow-up. The highest increase in CRC risk was seen for both overweight and obesity (increase by 26% and 42%, respectively) when 7 years of follow-up time were excluded. These risk increases are almost twice as high as the risk increases estimated when no follow-up years were excluded. Similarly, PAFs for overweight and obesity were estimated to be below 9% with follow-up times up to 8 years, 11.3% with the full period of follow-up (up to 13 years), but the estimate further increased to 19.0% after exclusion of the initial 7 years of follow-up.
Table 4 summarises results of the subgroup analyses, which show similar patterns as those observed in the main analyses. In all subgroups, the lowest increase in the risk of CRC or even no increase at all was observed for overweight and obesity during the initial 7 years of follow-up, and the increase in the CRC risk was higher when the initial 7 years of follow-up were excluded compared to when all the follow-up data were used. Overall, associations were stronger for older (≥50 years) than for younger (<50 years) participants, for men than for women, for former and current smokers than for never smokers, and for those with no history of bowel cancer screening. However, for some of the subgroup analyses, case numbers were rather low, which resulted in broad confidence intervals for some of the HRs. Point estimates of some HRs were also non-significantly lower than 1, which explains the apparent negative PAFs for some categories. Statistically significant interactions were observed for sex (Pinteraction < 0.001) and history of bowel cancer screening (Pinteraction = 0.04).
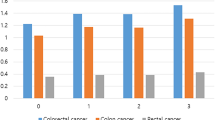
Table 5 shows the site-specific analyses for the association between overweight and obesity with the risk of colon, proximal colon, distal colon and rectal cancer separately. The association was stronger for colon cancer compared to rectal cancer in general, but heterogeneity by tumour site was not statistically significant (Pheterogeneity = 0.11), and quite similar for proximal and distal colon cancer (Pheterogeneity = 0.17). Furthermore, the risk increase was much stronger in the later years of follow-up than in the initial years of follow-up, with PAF estimates for both proximal and distal colon cancer reaching 24% in the later years of follow-up.
Discussion
In this large population-based cohort study, associations between excess weight and CRC risk were very weak or even absent during the initial years of follow-up, increased with increasing the length of follow-up, and were most pronounced in analyses based on long-term follow-up data in which the initial years of follow-up were excluded. These patterns are consistent with and support the hypothesis that the excess weight–CRC risk association may be substantially stronger than suggested by previous evidence from epidemiological studies with shorter follow-up or paying less attention to exclude or minimise bias due to prediagnostic weight loss.
Weight loss is a common symptom among CRC patients at different stages of the disease including the pre-clinical sojourn time [22,23,24], with estimated mean duration of 3 to 6 years [14,15,16]. Underlying mechanisms include increased catabolism and systemic inflammation caused by the tumour, which can lead to a negative energy balance and cancer cachexia [25, 26]. Such weight loss results in migration of patients with higher BMI to lower BMI categories, which in turn may result in weaker or even inverse associations of overweight and obesity with CRC risk.
Although cancer related weight loss has been recognised as a potential source of bias in epidemiological studies linking excess weight with CRC risk, in a recent umbrella review we provided evidence that many studies have not taken this potential bias into account in their analyses [27]. For instance, among the 21 cohort studies included in a recent systematic review and meta-analysis [11] evaluating the BMI-CRC incidence association, 15 studies (71%) did not implement any sort of exclusion of the first years of follow-up in their main analysis [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], only one study excluded more than the first year of follow-up [43], and, where conducted, sensitivity analyses mostly just excluded the initial 1 or 2 years of follow-up [29,30,31,32, 34, 36,37,38, 41]. In agreement with our findings, higher risk estimates were obtained in the sensitivity analyses in most cases. However, most studies were based on much smaller cohorts and none had provided a similarly comprehensive analysis of the potential impact of prediagnostic weight loss. Even though we aimed to minimise bias from prediagnostic weight loss, our results may still underestimate the true impact of excess weight. Even stronger effect estimates would be expected when life-time exposure to excess weight could be taken into account as recently demonstrated in a large study from Germany [44].
In a recent study from UK, the PAF of overweight and obesity was estimated as 13.3% for men and 5.6% for women [45]. These estimates, which were based on combining summary risk estimates from cohort studies and nationally representative survey data on prevalence of overweight and obesity in the UK, are comparable to our results (15.0% and 6.2%) with no exclusion of follow-up years, which according to our findings, is likely an underestimation of the CRC burden attributable to overweight and obesity. In another study, PAF estimates reported by sex and cancer site in 30 European countries [5], were lower for all countries compared to our results from UK, which may reflect both lower previous risk estimates and lower prevalence of overweight and obesity in most European countries compared to the UK.
In line with the results from recent systematic reviews [2, 46, 47] and the World Cancer Research Fund report [48], we observed weaker associations of overweight and obesity with CRC incidence for women compared to men, regardless of the length of follow-up included in the analysis, and therefore, lower PAFs of overweight and obesity were calculated for women. The Women’s Health Initiative trial [49], the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort [50], and other studies and reviews [51,52,53], have pointed out the potential role of hormone replacement therapy (HRT) in post-menopausal women as an effect modifier in the BMI-CRC association, and women receiving HRT have been reported to be at lower risk of CRC. Endogenous sex-hormones have also been suggested as an explanation for the difference seen between men and women regarding CRC incidence, however the evidence has remained inconsistent. The weaker association due to HRT use might also be of relevance in our study population in which 37.5% of women reported receiving HRT at baseline.
A major strength of our study is use of the very large and comprehensive database of the UK Biobank with long-term follow-up and considerable numbers of participants and CRC cases, which allowed us to adjust for a broad range of covariates and to estimate risks at high levels of precision, even in the analyses restricted to shorter time windows of follow-up, and in subgroup and site-specific analyses. Most importantly, the BMI variable provided by the UK Biobank is based on highly standardised measurements rather than self-reported weight and height and is therefore not affected by reporting bias.
Our study also has limitations. First, due to the observational nature of the study, residual confounding may still exist despite adjustment for a large set of covariates. Second, despite the overall large number of participants and CRC cases, case number limitations still made reasonably precise risk estimates for underweight participants and obesity subclasses infeasible, which therefore had to be combined in most of our analyses. Third, our analyses exclusively focused on BMI as the most commonly used measure of excess weight in epidemiological studies. Central obesity measures such as waist circumference and waist-to-hip ratio (WHR) may be even stronger and more robust predictors of incident CRC than BMI [54,55,56] and should be addressed in future research. Fourth, the UK Biobank population mostly consists of white European participants and therefore, the results might not be generalisable to other populations.
Our analyses, based on the very large UK Biobank cohort, provide evidence that excess weight may account for a substantially larger share of the CRC burden than previously suggested. Future studies should pay more attention to avoid underestimation of the role of overweight and obesity due to prediagnostic weight loss by more rigorously considering timing of exposure measurement and taking cumulative life-time exposure rather than weight at a single point of time into account. Given the high and continuously increasing prevalence of overweight and obesity, this burden is expected to further increase and, along with demographic aging, will further accelerate the expected rise in numbers of CRC cases in many countries around the globe. Enhanced efforts to cope with the obesity epidemic will be crucial for more effective prevention of CRC as well as many other excess weight related cancers and diseases.
Data availability
Data was re-used with the permission of the UK Biobank. This work used data provided by patients and collected by the NHS as part of their care and support. The UK Biobank is an open-access resource and bona fide researchers can apply to use the UK Biobank dataset by registering and applying at https://www.ukbiobank.ac.uk/enableyourresearch/apply-for-access. Further information is available from the corresponding author upon request.
Code availability
The code used to analyse the data and generate the results in this study is available from the UK Biobank resource for all researchers upon registration to access the UK Biobank data at https://www.ukbiobank.ac.uk/enableyourresearch/apply-for-access.
References
Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477 https://doi.org/10.1136/bmj.j477
Fang X, Wei J, He X, Lian J, Han D, An P, et al. Quantitative association between body mass index and the risk of cancer: a global meta-analysis of prospective cohort studies. Int J Cancer. 2018;143:1595–603. https://doi.org/10.1002/ijc.31553
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660
World Health Organization. Obesity and overweight 2021. Available at https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight accessed on 25 Nov 2021.
Renehan AG, Soerjomataram I, Tyson M, Egger M, Zwahlen M, Coebergh JW, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126:692–702. https://doi.org/10.1002/ijc.24803
Gu MJ, Huang QC, Bao CZ, Li YJ, Li XQ, Ye D, et al. Attributable causes of colorectal cancer in China. BMC Cancer. 2018;18:38 https://doi.org/10.1186/s12885-017-3968-z
Vajdic CM, MacInnis RJ, Canfell K, Hull P, Arriaga ME, Hirani V, et al. The future colorectal cancer burden attributable to modifiable behaviors: a pooled cohort study. JNCI Cancer Spectrum. 2018;2. https://doi.org/10.1093/jncics/pky033
Cho S, Shin A. Population attributable fraction of established modifiable risk factors on colorectal cancer in Korea. Cancer Res Treat. 2021;53:480–6. https://doi.org/10.4143/crt.2019.742
Bardou M, Rouland A, Martel M, Loffroy R, Barkun AN, Chapelle N. Review article: obesity and colorectal cancer. Aliment Pharmacol Ther. 2022;56:407–18. https://doi.org/10.1111/apt.17045
Lei X, Song S, Li X, Geng C, Wang C. Excessive body fat at a young age increases the risk of colorectal cancer: a systematic review and meta-analysis. Nutr Cancer. 2021;73:1601–12. https://doi.org/10.1080/01635581.2020.1804951
Zhang C, Cheng Y, Luo D, Wang J, Liu J, Luo Y, et al. Association between cardiovascular risk factors and colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. EClinicalMedicine. 2021;34:100794 https://doi.org/10.1016/j.eclinm.2021.100794
Slawinski CGV, Barriuso J, Guo H, Renehan AG. Obesity and cancer treatment outcomes: interpreting the complex evidence. Clin Oncol (R Coll Radiol). 2020;32:591–608. https://doi.org/10.1016/j.clon.2020.05.004
Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93:399–405. https://doi.org/10.1038/sj.bjc.6602714
Launoy G, Smith TC, Duffy SW, Bouvier V. Colorectal cancer mass-screening: estimation of faecal occult blood test sensitivity, taking into account cancer mean sojourn time. Int J Cancer. 1997;73:220–4.
Brenner H, Altenhofen L, Katalinic A, Lansdorp-Vogelaar I, Hoffmeister M. Sojourn time of preclinical colorectal cancer by sex and age: estimates from the German national screening colonoscopy database. Am J Epidemiol. 2011;174:1140–6. https://doi.org/10.1093/aje/kwr188
Sung NY, Jun JK, Kim YN, Jung I, Park S, Kim GR, et al. Estimating age group-dependent sensitivity and mean sojourn time in colorectal cancer screening. J Med Screen. 2019;26:19–25. https://doi.org/10.1177/0969141318790775
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779 https://doi.org/10.1371/journal.pmed.1001779
UK Biobank Coordinating Centre. UK Biobank: Protocol for a large-scale prospective epidemiological resource 2007. Available at https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf accessed on 08 Apr 2022.
WHO Consultation on Obesity (1999: Geneva, Switzerland) & World Health Organization. (2000). Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series 2000;894:i–xii, 1–253.
Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71:114–20. https://doi.org/10.1080/02701367.2000.11082794
Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–41.
Nicholson BD, Hamilton W, O’Sullivan J, Aveyard P, Hobbs FR. Weight loss as a predictor of cancer in primary care: a systematic review and meta-analysis. Br J Gen Pract. 2018;68:e311–e22. https://doi.org/10.3399/bjgp18X695801
Nicholson BD, Hamilton W, Koshiaris C, Oke JL, Hobbs FDR, Aveyard P. The association between unexpected weight loss and cancer diagnosis in primary care: a matched cohort analysis of 65,000 presentations. Br J Cancer. 2020;122:1848–56. https://doi.org/10.1038/s41416-020-0829-3
Nicholson BD, Aveyard P, Price SJ, Hobbs FR, Koshiaris C, Hamilton W. Prioritising primary care patients with unexpected weight loss for cancer investigation: diagnostic accuracy study. BMJ. 2020;370:m2651 https://doi.org/10.1136/bmj.m2651
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. https://doi.org/10.1016/s1470-2045(10)70218-7
Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–9. https://doi.org/10.1038/nrclinonc.2012.209
Mandic M, Li H, Safizadeh F, Niedermaier T, Hoffmeister M, Brenner H. Is the association of overweight and obesity with colorectal cancer underestimated? An umbrella review of systematic reviews and meta-analyses. Eur J Epidemiol. 2023;38:135–44. https://doi.org/10.1007/s10654-022-00954-6
Engeland A, Tretli S, Austad G, Bjørge T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control. 2005;16:987–96. https://doi.org/10.1007/s10552-005-3638-3
Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–54. https://doi.org/10.1200/jco.2005.11.726
Larsson SC, Rutegård J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer. 2006;42:2590–7. https://doi.org/10.1016/j.ejca.2006.04.015
Lukanova A, Björ O, Kaaks R, Lenner P, Lindahl B, Hallmans G, et al. Body mass index and cancer: results from the Northern Sweden Health and Disease Cohort. Int J Cancer. 2006;118:458–66. https://doi.org/10.1002/ijc.21354
Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134 https://doi.org/10.1136/bmj.39367.495995.AE
Song YM, Sung J, Ha M. Obesity and risk of cancer in postmenopausal Korean women. J Clin Oncol. 2008;26:3395–402. https://doi.org/10.1200/jco.2007.15.7867
Bassett JK, Severi G, English DR, Baglietto L, Krishnan K, Hopper JL, et al. Body size, weight change, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2978–86. https://doi.org/10.1158/1055-9965.Epi-10-0543
Renehan AG, Flood A, Adams KF, Olden M, Hollenbeck AR, Cross AJ, et al. Body mass index at different adult ages, weight change, and colorectal cancer risk in the National Institutes of Health-AARP Cohort. Am J Epidemiol. 2012;176:1130–40. https://doi.org/10.1093/aje/kws192
Li H, Yang G, Xiang YB, Zhang X, Zheng W, Gao YT, et al. Body weight, fat distribution and colorectal cancer risk: a report from cohort studies of 134255 Chinese men and women. Int J Obes (Lond). 2013;37:783–9. https://doi.org/10.1038/ijo.2012.152
Steins Bisschop CN, van Gils CH, Emaus MJ, Bueno-de-Mesquita HB, Monninkhof EM, Boeing H, et al. Weight change later in life and colon and rectal cancer risk in participants in the EPIC-PANACEA study. Am J Clin Nutr. 2014;99:139–47. https://doi.org/10.3945/ajcn.113.066530
Lu Y, Ness-Jensen E, Hveem K, Martling A. Metabolic predispositions and increased risk of colorectal adenocarcinoma by anatomical location: a large population-based cohort study in Norway. Am J Epidemiol. 2015;182:883–93. https://doi.org/10.1093/aje/kwv141
Hanyuda A, Cao Y, Hamada T, Nowak JA, Qian ZR, Masugi Y, et al. Body mass index and risk of colorectal carcinoma subtypes classified by tumor differentiation status. Eur J Epidemiol. 2017;32:393–407. https://doi.org/10.1007/s10654-017-0254-y
Andreasson A, Hagström H, Sköldberg F, Önnerhag K, Carlsson AC, Schmidt PT, et al. The prediction of colorectal cancer using anthropometric measures: a Swedish population-based cohort study with 22 years of follow-up. United European Gastroenterol J. 2019;7:1250–60. https://doi.org/10.1177/2050640619854278
Bjørge T, Häggström C, Ghaderi S, Nagel G, Manjer J, Tretli S, et al. BMI and weight changes and risk of obesity-related cancers: a pooled European cohort study. Int J Epidemiol. 2019;48:1872–85. https://doi.org/10.1093/ije/dyz188
Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5:37–44. https://doi.org/10.1001/jamaoncol.2018.4280
Moore LL, Bradlee ML, Singer MR, Splansky GL, Proctor MH, Ellison RC, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obes Relat Metab Disord. 2004;28:559–67. https://doi.org/10.1038/sj.ijo.0802606
Li X, Jansen L, Chang-Claude J, Hoffmeister M, Brenner H. Risk of colorectal cancer associated with lifetime excess weight. JAMA Oncol. 2022;8:730–7. https://doi.org/10.1001/jamaoncol.2022.0064
Goon S, Kim H, Giovannucci E. Population attributable risk for colorectal and breast cancer in England, Wales, Scotland, Northern Ireland, and the United Kingdom [version 2; peer review: 2 approved]. 2022;3. https://doi.org/10.12688/amrcopenres.12980.2
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. https://doi.org/10.1016/s0140-6736(08)60269-x
Abar L, Vieira AR, Aune D, Sobiecki JG, Vingeliene S, Polemiti E, et al. Height and body fatness and colorectal cancer risk: an update of the WCRF-AICR systematic review of published prospective studies. Eur J Nutr. 2018;57:1701–20. https://doi.org/10.1007/s00394-017-1557-1
Continuous Update Project Expert Report 2018. Body fatness and weight gain and the risk of cancer: World Cancer Research Fund/American Institute for Cancer Research; 2018. Available at https://www.wcrf.org/wp-content/uploads/2021/01/Body-fatness-and-weight-gain_0.pdf accessed on 20 Nov 2021.
Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. https://doi.org/10.1056/NEJMoa032071
Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjønneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:920–31. https://doi.org/10.1093/jnci/djj246
Hoffmeister M, Raum E, Winter J, Chang-Claude J, Brenner H. Hormone replacement therapy, body mass, and the risk of colorectal cancer among postmenopausal women from Germany. Br J Cancer. 2007;97:1486–92. https://doi.org/10.1038/sj.bjc.6604066
Hang D, Shen H. Sex hormone and colorectal cancer: the knowns and unknowns. Cancer Epidemiol Biomarkers Prev. 2021;30:1302–4. https://doi.org/10.1158/1055-9965.Epi-21-0472
Wele P, Wu X, Shi H. Sex-dependent differences in colorectal cancer: with a focus on obesity. Cells. 2022;11:3688 https://doi.org/10.3390/cells11223688
Ortega LS, Bradbury KE, Cross AJ, Morris JS, Gunter MJ, Murphy N. A prospective investigation of body size, body fat composition and colorectal cancer risk in the UK Biobank. Sci Rep. 2017;7:17807 https://doi.org/10.1038/s41598-017-17997-5
Hashemi Madani N, Etemadi A, Nalini M, Poustchi H, Khajavi A, Mirzazade E, et al. Obesity and incident gastrointestinal cancers: overall body size or central obesity measures, which factor matters? Eur J Cancer Prev. 2021;30:267–74. https://doi.org/10.1097/cej.0000000000000657
Mili N, Paschou SA, Goulis DG, Dimopoulos MA, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: pathophysiological and therapeutic associations. Endocrine. 2021;74:478–97. https://doi.org/10.1007/s12020-021-02884-x
Acknowledgements
We thank UK Biobank participants. This research has been conducted using the UK Biobank Resource under application number 66591.
Funding
The UK Biobank was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish Government and the Northwest Regional Development Agency. It has also had funding from the Welsh Government, British Heart Foundation, Cancer Research UK, Diabetes UK, and National Institute for Health Research (NIHR). UK Biobank is supported by the National Health Service (NHS). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
HB designed the study. FS analysed the data and with HB drafted the manuscript. HB, FS, MM, DP and TN interpreted the data. All authors have contributed substantially to revising the manuscript. All authors approved the version of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The UK Biobank was approved by the North West Multi center Research Ethics Committee (MREC) as a Research Tissue Bank (RTB) approval (renewed approval in 2021:21/NW/0157).
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Safizadeh, F., Mandic, M., Pulte, D. et al. The underestimated impact of excess body weight on colorectal cancer risk: Evidence from the UK Biobank cohort. Br J Cancer 129, 829–837 (2023). https://doi.org/10.1038/s41416-023-02351-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02351-6