Abstract
Breast cancer (BrCa) is the leading cause of cancer incidence and mortality in women worldwide. While BrCa treatment has been shown to be highly successful if detected at an early stage, there are few effective strategies to treat metastatic tumours. Hence, metastasis remains the main cause in most of BrCa deaths, highlighting the need for new approaches in this group of patients. Immunotherapy has been gaining attention as a new treatment for BrCa metastasis and the kynurenine pathway (KP) has been suggested as one of the potential targets. The KP is the major biochemical pathway in tryptophan (TRP) metabolism, catabolising TRP to nicotinamide adenine dinucleotide (NAD+). The KP has been reported to be elevated under inflammatory conditions such as cancers and that its activity suppresses immune surveillance. Dysregulation of the KP has previously been reported implicated in BrCa. This review aims to discuss and provide an update on the current mechanisms involved in KP-mediated immune suppression and cancer growth. Furthermore, we also provide a summary on 58 studies about the involvement of the KP and BrCa and five clinical trials targeting KP enzymes and their outcome.
Similar content being viewed by others
Breast cancer
Breast cancer (BrCa) is the leading cause of cancer incidence worldwide with 2.26 million cases in 2020 alone or 11.7% of total cancer cases [1]. It is also the fourth leading cause of cancer-related deaths with 685,000 deaths in 2020 or 6.9% of total cancer-related death [1]. Although BrCa leads cancer incidence worldwide, it is generally curable in a majority of diagnosed patients [1, 2]. This is partly due to a combination of early detection and advances in treatment [1, 3]. Treatment of BrCa is usually guided by molecular characteristics of the tumour [4]. Four of the most common subtypes [5] include: luminal-A [6], luminal-B [7, 8], human epidermal growth factor receptor 2 (HER2)-enriched [9] and triple-negative (TNBC) [10] as summarised in Fig. 1. Luminal-A and B subtypes [11] have the highest 5-year cancer survival rates followed by HER2-enriched [9, 12], and TNBC [13].
BrCa can be categorised into four major subtypes based on its molecular characteristics: Luminal-A, luminal-B, HER2-enriched and TNBC. Up to 30% and 20% of total BrCa cases are luminal-A and luminal-B subtype, respectively. Both subtypes have the highest survival rate of up to 85% due to the availability of targeted treatments. HER2-enriched subtype accounts for 20% of total cases and has a lower 5-year survival rate of 75%. Finally, it is the TNBC that makes up to 15% of total cancer cases and has the poorest 5-year survival rate of 62%. This is due to the absence of targeted treatments that the other subtypes have.
Despite the combination of advancements in early screening and detection and targeted therapy as highlighted above, metastasis remains a cause of more than 90% of BrCa deaths [14]. Immunotherapy has emerged as one of the cancer treatment options to contain or slow down the spread of cancer [15]. One treatment approach involves the boosting of the host’s suppressive ability against cancer cells as demonstrated by immune checkpoint inhibitors including anti-cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and programmed death -1 (PD-1; pembrolizumab) and PD ligand-1 (PD-L1; atezolizumab) [16]. While these treatments have improved overall survival in patients with metastatic melanoma [17] and non-small cell lung cancer[18], the overall response rates to immunotherapy with pembrolizumab or atezolizumab in metastatic TNBC BrCa are still modest (between 5% and 25% [19,20,21]. Combinatory regimens of these immune checkpoint inhibitors and chemotherapy are currently under investigation as detailed in the review by Thomas et al. [22]. The modest response rates in TNBC BrCa to immune checkpoint inhibitors implies the involvement of different immune evasion mechanisms. The KP has been suggested as the alternate mechanism that tumour cells can use to suppress local immune surveillance to facilitate growth and spread [23].
The KP
TRP is one of the essential amino acids that is not endogenously synthesised and needs to be obtained from the diet for protein synthesis [24]. Once introduced with food, TRP is absorbed from the gut and released into the bloodstream, where it is transported to various tissues and cells [25]. The diet-derived TRP has distinct metabolic fates - while up to 30% of TRP and a smaller portion of TRP is used as a precursor for protein synthesis [26] and the production of neurotransmitter serotonin and neuromodulator/neurohormone melatonin respectively, most TRP is metabolised through the KP [27] (Fig. 1). One of the main physiological roles of the KP is to generate NAD + through the KP. Three different enzymes can catabolise TRP into the KP first metabolic step: tryptophan 2,3 dioxygenase (TDO) [28]; indoleamine 2,3 dioxygenase 1 (IDO1) [29] and indoleamine 2,3 dioxygenase 2 (IDO2) [30,31,32]. These enzymes catalyse the same enzymatic step, although each of them has distinct inducers and patterns of tissue expression.
TDO is expressed mainly in the liver [33] and other selected organs such as brain [34], embryonic tissues [35] and placenta [36]. TDO is activated by its substrate TRP or by glucocorticoids [37]. TDO can be inhibited by oestrogens and progesterone [38], as well as by NAD/NADH by a negative feedback loop [39]. IDO1 is expressed virtually in all major organs [40] and monocyte lineage immune cells [41,42,43]. The transcription and expression of IDO1 is rapidly upregulated by pro-inflammatory cytokines such as interferon-γ (IFN-γ) tumour necrotic factor-α (TNF-α) [44] and other pro-inflammatory mediators, such as lipopolysaccharide, amyloid peptides, and viral proteins among others [45]. Conversely, IDO1 is inhibited by its substrate TRP [46], by anti-inflammatory cytokines such as interleukin (IL)-10 and IL-4, as well as by the antioxidant enzyme superoxide dismutase [47]. IDO2 expression is detected in neuronal cells of the cerebral cortex, hepatocytes, the bile duct and dendritic cells [48, 49]. IDO2 is a less well-studied enzyme, and its inducer and biological roles remain unclear [30,31,32].
As mentioned above, TRP can be metabolised into the KP producing NAD+ product, or into other KP intermediates in a multi-branched arrangement, mediated by enzymes and their inducers acting at the different levels of the pathway [50, 51] (Fig. 2). Briefly, TRP is metabolised by TDO, IDO1 or IDO2 into kynurenine (KYN). KYN is a central metabolite in the KP. It can be further metabolised by three different enzymes: kynurenine aminotransferase (KATs), kynureninase (KYNU) or kynurenine monooxygenase (KMO) to produce the intermediates kynurenic acid (KYNA), anthranilic acid (AA) and 3-hydroxykynurenine (3HK), respectively (Fig. 2).
TRP is a precursor for protein synthesis and neurotransmitter serotonin and neuromodulator/neurotransmitter melatonin. The majority of the dietary TRP is catabolised through the KP. TDO, one of the rate-limiting enzymes of the pathway, is activated by elevated glucocorticoids, KYN, 3HK and TRP and inhibited by NADH. IDO is another rate-limiting enzyme of the KP, and it is activated in the presence of pro-inflammatory mediators such as TNF-α, IFN-γ, IFN-α, IFN-β and LPS. On the other hand, IDO is inhibited by SOD and anti-inflammatory mediators such as IL-4 and IL-10. Following the KP activation, TRP is metabolised into metabolites through specific enzymes catalysing each reaction in various cells/tissues. KYN, a central metabolite of the KP and can be catabolized into three different intermediates, KYNA, AA and 3HK by KATS, KYNU and KMO respectively. 3HK can further be converted to 3HAA by KYNU and then to 2-amino-3-carboxymuconate semialdehyde ACMS by HAAO. Alternatively, 3HK can also be metabolised by KATs into XA or into 3HKA. Additionally, the oxidation-reduction reaction can convert AA to 3HAA, while 3HAA by auto-oxidation can produce CA. The metabolite ACMS is positioned at a key junction of the pathway to either non-enzymatically form the metabolite QUIN, a precursor for de novo synthesis of NAD+ or converted to PIC by ACMSD.
The preferred catabolic route for KYN in the brain is to produce KYNA by KATs under normal physiological condition [52]. Due to the low affinity of KYN to KYNU, the conversion of KYN to AA only occurs when the concentration of KYN is considerably elevated [53]. The oxidation-reduction converts AA further to 3-hydroxyanthranilic acid (3HAA) [54]. The KMO route is more active in the peripheral tissues, particularly in the liver and kidney, and less active in the brain [55, 56]. The metabolite 3HK can then be subsequently converted to 3HAA by KYNU, to xanthurenic acid (XA) by KATs or even to 3-hydroxy-L-kynurenamine (3HKA) [57]. The metabolite 3HAA can give rise to cinnabarinic acid (CA) by spontaneous auto-oxidation [58] or to 2-amino-3-carboxymuconate semialdehyde (ACMS) by the enzyme 3-hydroxyanthranilate dioxygenase (HAAO). Next, ACMS can either non-enzymatically form quinolinic acid (QUIN), a vital precursor for de novo synthesis of NAD+ or picolinic acid (PIC) by 2-amino-3-carboxymuconate semialdehyde decarboxylase (ACMSD) [59].
As mentioned above, the KP has a major role in physiological processes through the supply of NAD+, a co-factor required in many biochemical processes. In some mammals, the KP can supply sufficient NAD+ in the absence of any dietary niacin intake [60]. Another established biological role for the KP is the regulation of the immune response [61]. It has been suggested to be involved in the maintenance of immunological tolerance and immune privilege in the eyes, brain, placenta, hair follicles and colon [62, 63].
Initially, the KP was simply thought to function as an immunomodulatory mechanism by allowing cells to deplete TRP from the intracellular pool or local microenvironment and, therefore, lead to the suppression of T-cell function and growth [64]. Accumulating evidence has demonstrated that KP-mediated immunoregulatory processes are also triggered by the biological activity of KP metabolites. Several KP metabolites are ligands of the aryl hydrocarbon receptor (AhR), a ligand-operated transcription factor. They have been shown to modify the behaviour of immune cells through a more tolerogenic phenotype [65, 66]. Several recent studies have suggested a link between the KP-dependent activation of AhR and the development and normal functioning of immune systems [65,66,67,68]. For instance, KP-mediated activation of AhR in intestinal epithelia is essential for developing and maintaining intestinal barrier function, a mechanism that, when disrupted, can contribute to inflammatory bowel diseases [69].
Roles of the KP in cancer
The involvement of the KP in cancer was first observed in early studies where cancer patients were reported to have higher KP activity [70, 71]. As compared to healthy controls, cancer patients were shown to have lower concentrations of TRP and higher concentrations of KP metabolites in their blood and urine. The pivotal study that provides the most substantial evidence of the KP involvement in cancer is the study by Muller et al. They demonstrated that combination therapy of a chemotherapeutic drug and an IDO1 inhibitor, 1-methyl-D-tryptophan (1MT), reduced the tumour size by 30% in a HER2-enriched mouse model. Furthermore, this treatment is highly dependent on a competent immune system [72]. Since the outcome of this study, the KP has emerged as a potential target to address cancer progression and metastasis [73]. The attention on KP and cancer is centred on its capacity to suppress local immune surveillance, and there are at least three different mechanisms (Fig. 3). As TRP is one of the critical components required for T-cell survival, a first mechanism involves the overactivation of IDO1 and/or TDO to deplete TRP within the local tumour microenvironment rapidly. Consequently, a TRP-stripped tumour microenvironment mediated by an overactive tumoral IDO1/TDO will induce mid-G1 phase arrest in T cells [74]. Elevated IDO1/TDO activity has been reported in tumour cells and blood sampled from patients with cancer, including glioblastoma [75, 76], colorectal cancer [77, 78], breast cancer [79, 80] and liver cancer [81]. This notion was supported by evidence of high IDO1 expression detected at metastatic sites and that the expression of IDO1 correlates positively with the immune-suppressive T regulatory population [82,83,84,85]. The association between the KP and various cancers indicates that the KP may play an important role in cancer progression.
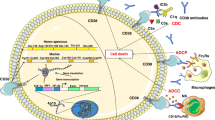
Three mechanisms are proposed for the suppression of local immune surveillance by the KP: (1) An overactive tumoral IDO1/ TDO can reduce the TRP availability in the tumour microenvironment and ultimately lead to T-cell proliferation arrest and increased ability of cancer cells to escape T-cell-dependent antitumor immunity. (2) KP metabolites 3HK, 3HAA and QUIN can suppress anti-tumour immunity. 3HK, 3HAA and QUIN induce apoptosis of anti-tumour immune cell populations such as CD4+ and CD8+ T via caspase activation, while 3HAA can induce CD4+ T-cell death via NF-κB inhibition. (3) The KP metabolites KYN, KYNA and CA are AhR ligands. Activation of the AhR on T cells reduces T-cell differentiation and activation. Activation of the AhR on the tumour cells induces an inflammatory positive autocrine feedback loop that enhance the production of AhR ligand and immunosuppressive KP metabolites.
The second immune suppression mechanism of the KP involves its metabolites and their interaction with immune T cells and tumours (Fig. 3). Notably, three KP metabolites 3HK, 3HAA and QUIN were shown to suppress anti-tumour immune cell populations. Of these KP metabolites, 3HAA has the most potent immune-suppressive capability because of the number of T-cell populations it interacts with. In vitro studies showed that 3HAA selectively induces apoptosis in CD4+ T-cell populations through the caspase activation pathway while inhibiting CD8+ T-cell expansion and activation [86, 87]. Additionally, 3HAA is capable of skewing T-cell differentiation towards regulatory T cells instead of anti-tumour T cells [88]. On the other hand, 3HK and QUIN were shown to induce cell death in CD4+ T cells [86, 89]. Apart from its immune-suppressive properties, QUIN was also shown to enhance glioblastoma cell survival when in excess. The enhanced cell survival may be due to the excess QUIN increasing the availability of NAD+ when catabolised. This NAD+ is then utilised in DNA repair, essentially reducing the apoptosis that was triggered by chemotherapeutic drugs [90].
A last immune-suppressive mechanism of the KP is mediated through the interaction between some KP metabolites and the ligand-activated transcription factor, the AhR. The AhR is expressed in all cell types, and its activation can trigger various biological pathways regulating the immune response and various cellular functions [91]. Other than KYN, KYNA, and cinnabarinic acid (CA), the following metabolites indole-3-pyruvic acid (I3P), indole-3-acetic acid, and indole-3-carboxaldehyde are also known ligands to the AhR [92]. Interestingly, the type of biological pathway activated depends on the cell type on which AhR is activated. T-cell differentiation and activation is reduced when AhR is activated on the T cells by KYN [76]. Activation of AhR on the tumour by KYN induces an inflammatory positive autocrine feedback loop (IDO1-AhR-IL-6-STAT3 signalling pathway) to enhance tumour growth [93]. This pro-inflammatory feedback loop can also be triggered by KYNA [94].
The KYNA triggered observed feedback loop is produced by the activation of the Interleukin-4 induced gene 1 (IL4I1) rather than the KP. Sadik et al. showed that melanoma has high expression of IL4I1 producing KYNA, and KYNA activation of the AhR enhanced tumour cell motility and T-cell proliferation [95]. Elevated of IL4I1 activity is not affected by IDO1 inhibition, suggesting that this new mechanism may be leading to IDO1 and/or immune checkpoint inhibition resistance.
Involvement of the KP in BrCa
We conducted a search on PubMed using the keywords “kynurenine” and “breast cancer” and we found 58 studies that looked at the KP in BrCa. These studies and their outcomes are summarised in Table 1.
The possibility that TRP metabolism may have a role in BrCa was alluded to in early studies from the 1970’s by measurement of 3HAA, 3HK, AA, KYN, KYNA and XA in the urine of patients following ingestion of TRP [96,97,98,99]. Two studies showed that ~50% of patients with BrCa have higher rates of KP metabolism as compared to controls [96, 98]. This skewed observation of BrCa patients with elevated KP metabolism could be due to the distribution of BrCa subtypes in their study cohort [79] and not attributed to the ethnicity of the patients as this study showed no difference in KP metabolism between two ethnic groups [97]. A later study reported that patients with soft tissue metastases have a higher level of KP metabolism as compared to patients with bone metastases due to higher concentration of 3HK, XA and 3HAA in their urine samples [99]. Despite these findings from the 1970’s, no further studies were conducted until the early 2000’s, when research delved deeper into the regulation of the KP in BrCa and the potential role of KP metabolites and enzymes in the pathology of BrCa.
The advancements in analytical chemistry and molecular biology techniques may have led to an increased number of studies on the KP in BrCa after the year 2000. In particular, analytical chemistry techniques such as high-performance liquid chromatography (HPLC) have provided high-resolution separation and quantitation of components from a biological matrix with greater accuracy, sensitivity and reproducibility of results, which has made it an appropriate method to quantify chemical constituents in biological samples [100]. HPLC has emerged as a key technique used in the majority, if not all of the studies conducted after 2000 that explore the impact of IDO1 in BrCa by direct assessment of the pathway activity using the blood of patients or cell cultures. The common approach to measure IDO1 activity is by quantifying the primary substrate and immediate product of IDO1 enzymatic reaction. The activity of the enzyme can be calculated by a ratio of product and substrate and can be readily measured now from blood using simple ELISA kits (ImmuSmol). Blood collection is routinely performed, safe and inexpensive. However, the quantification of downstream KP metabolites require specialised and often large and expensive analytic equipment. Another limitation of this quantification methodology is that the ratio is a systemic measurement instead of a snapshot of the tumour environment. The KYN/TRP ratio is not specific only to IDO1 but also includes the activity of TDO (Fig. 2). These limitations can be addressed by quantifying the expression of IDO1 enzyme in tumour tissue using immunohistochemistry and/or the flow cytometry. The KYN/TRP ratio is the preferred approach as the latter techniques are usually time consuming and expensive. Both techniques also require surgery and the removal of tumour tissue from patients, which is more invasive when compared to blood collection. While this provides accurate levels and cellular localisation of IDO1 expression in the tissues, it does not provide information if this enzyme activity is inhibited or not. Hence, studies usually combine both techniques to provide an accurate overview of IDO1 activity in the tumour and to assess if its activity is inhibited when an inhibitor is applied.
Since the early 2000’s, there were 40 studies examining the role of IDO1 in BrCa that includes the impact of chemotherapy on IDO1 activity and inhibition of IDO1 itself. As noted above, IDO1 is a significant rate-limiting enzyme that regulates the production of KP metabolites and is generally highly expressed in multiple human cancers [101]. Although most of the studies reported a positive correlation between BrCa progression and activity of IDO1 [102,103,104,105,106,107,108,109,110,111,112,113,114,115], one study showed the opposite observation [114] while another reported no significant differences in the expression of IDO1 between cancerous and non-cancerous tissues [116]. Inconsistent observations on the activity of IDO1 was also reported in patients with metastatic BrCa, even though these observations derived from studies that were conducted by the same research group. Their early study reported that expression of IDO1 positively correlates with the number of metastatic lesions [105] while their later studies showed the IDO1 activity was lower in patients with high number of metastatic lesions as compared to patients with no or lower number of metastatic lesions [106, 117]. The observed discrepancies could be due to the distribution of BrCa subtypes in their cohort as the KP was found to be highly elevated in HER2-enriched and TNBC but not luminal [79].
Interestingly, chemotherapy treatment reduced the activity of IDO1 in serum samples of BrCa patients and animal model study [106, 108, 118,119,120,121,122]. This suggests that the IDO1 may play a role in either tumour growth or tumour environment in BrCa. Given the evidence that IDO1 promotes T-cell anergy and suppresses tumour control [123], studies were carried out to examine the potential of inhibiting IDO1 to reduce tumour growth to unravel its role in BrCa progression. Results from in vitro, in vivo and clinical studies have revealed that the inhibition of IDO1 led to tumour suppression [122, 124,125,126,127,128,129]. This suppression of tumour is associated with an increased production of T cells such as CD4 + and CD8 + cells as well as an increased apoptosis of cancer cells [126, 127]. Intriguingly, a study showed that the efficacy of IDO1 inhibition is dependent on the activity of haem-oxygenase-1 (HO-1) [129]. HO-1 is a rate-limiting enzyme that controls the haem catabolism [130] and inactivity of both enzymes was suggested to release all available haem for the cancer cells to proliferate.
The above studies support the notion that IDO1 is elevated in tumours to suppress immune surveillance and favour tumour growth, suggesting IDO1 as a potential therapeutic target to treat BrCa. It is also important to note that there were studies showing either an increase in IDO1 activity [131,132,133] or no difference in IDO1 activity [134, 135] after receiving chemotherapy such as paclitaxel, Mohs paste or surgery. While there is limited information on the patient cohort, potential explanations for these different observations could be due to proportion of the subtypes of BrCa and/or the percentage of patients with IDO1 overexpressing-tumours enroled in these studies. Considering that there are also other rate-limiting enzymes besides IDO1, recent studies have examined TDO, and the KP downstream enzyme KMO and their interactions with other pathways. Five studies have reported that TDO levels have a positive correlation with worse overall survival, increased disease grade and invasion/migration capability [136]. Additionally, the expression of TDO is often detected concomitantly with the expression of IDO1 [82, 116, 137,138,139]. Furthermore, the expression of TDO showed a strong correlation with AhR and has been associated with enhanced tumour cell migration [82, 138,139,140]. KMO was reported to be elevated in the TNBC subtype and was associated with worse survival and malignant characteristics such as node-positive status. Invasive BrCa has been shown to have the highest KMO amplification among all human cancers [79, 141,142,143].
Clinical trials of IDO1 inhibitors in BrCa patients
IDO1 has emerged as an attractive pharmacological target as the enzyme has been well characterised and has high affinity for TRP among the small number of TRP catabolising enzymes (TDO, IDO2 and TPH). Given that it is the rate-limiting enzyme, inhibition of its activity may potentially block or reduce the activity of the whole KP, and its inhibitory action can be measured indirectly in the blood. As most of human cancers, including BrCa, overexpress IDO1, the validation of IDO1 inhibitor efficacy in enhancing anti-tumour immune activity could be translated for clinical use. With the positive outcome from the demonstrated efficacy of limiting tumour growth with combination treatment of IDO1 inhibition and chemotherapy drug(s) in in vivo and in vivo studies, 97 different clinical trials have been reported in ClinicalTrials.gov examining combination therapy in patients with cancers [144]. Among them are six clinical trials involving patients with BrCa; the various IDO1 inhibitors examined were epacadostat, indoximod and navoximod/GDC-0919 [144].
Epacadostat
Epacadostat has been shown to have the greatest efficacy in inhibiting IDO1 activity [145, 146] and in shifting the immune population towards tumour targeting CD8 + T cells [147]. The efficacy of epacadostat is based on preclinical studies that demonstrated it to have >100-fold selectivity for IDO1 as compared to other IDO1 inhibitors mentioned here. The administration of Epacadostat to tumour bearing syngeneic mice inhibited kynurenine production by ~90% in both tumour and plasma [146]. A solitary published trial, NCT02178722, is a phase I/II clinical trial that examined the efficacy of combining epacadostat with pembrolizumab to improve disease outcomes for patients with advanced solid tumours [148, 149]. In a phase I dosage escalation study, three of the 62 enroled patients were patients with TBNC, and only one achieved stable disease at the end of the phase. Within the cohort, tumour tissue from 22 participants were assessed for IDO1 expression. The assessment was carried out using the RNAscope technology and a scoring histoscore of more or equal to 5 was IDO1 positive. 13 patients were shown to have high IDO1 activity in tumour-infiltrating immune cells while 9 were evaluated to be negative as their histoscore was below the arbitrary cut-off value. The IDO1 status for the remaining patients remained unknown. Based on an encouraging clinical profile of more than 50% inhibition of IDO1 activity, 100 mg was chosen as the optimal dose for the Phase II study [148]. A total of 382 patients were enroled, with a selected schedule of 100 mg epacadostat with 200 mg of pembrolizumab. Thirty-six of the patient cohort were TNBC. Among the BrCa patients, one patient had a complete response, one showed very good response, two only minor response, while seven patients showed stable disease and 19 patients exhibited disease progression. Evaluation of disease progression could not be conducted in six patients. Overall, 51% of patients in the Phase II study experienced treatment-related adverse events; up to 15% of these adverse events were grade 3 or higher. At the conclusion of the study, TNBC was not considered for phase III inclusion [149]. NCT03328026, is a phase I/II trial currently recruiting patients to evaluate the combination treatment of SV-BR-1-GM with INCMGA00012 and epacadostat in metastatic or locally recurrent BrCa patients. This trial is currently recruiting and is expected to conclude on 31 December 2022.
Indoximod
Indoximod is the most studied IDO1 inhibitor in in vivo studies. It mimics TRP in reversing IDO1-mediated inhibition of the mammalian target of rapamycin complex 1 (mTORC1) [150, 151]. mTORC1 is one of the major signalling pathways regulating cell responses to intracellular nutrients such as TRP [152]. Two completed trials have examined Indoximod in the treatment of BrCa patients. NCT01792050 is a Phase II trial that observed the efficacy of a combination treatment of indoximod with taxane chemotherapy in patients with metastatic BrCa [153]. Recruitment criteria included HER2 negative tumours, normal organ function with the ability to receive taxane chemotherapy (paclitaxel or docetaxel), no history of autoimmune disease and no prior history of immunotherapy. A total of 169 patients were enroled in the trial. Results showed that there was no significant difference in the occurrence of toxic events and progression free survival between the combination therapy and single treatment arm (6.8 vs 9.5 months, respectively). Furthermore, there was no significative difference in response rates between the groups (40% and 37%) or overall survival (19.5 vs 20.6 months). Due to these lack of efficacy the study was discontinued prematurely. In determining its potential to be used as a predictor of response to indoximod, 52 archived tumour samples from the above reported study were stained for their IDO1 expression using IHC staining on these tumour samples. A cut-off score of 16.5 was derived as the median from previously stained samples. This score was used to classify the IDO1 expression as either high or low [153].
Although patients with high IDO1 expressing tumours (n = 30) were found to have longer progression free survival and overall survival than patients with low IDO1 expressing tumours (n = 22), the differences were not statistically significant. A later phase I/II trial, NCT01042535, examined the efficacy of combining indoximod with an adenovirus-p53 transduced dendritic cell vaccine in the treatment of patients with metastatic BrCa. Of the 39 patients enroled, 22 patients who obtained more than two cycles of post vaccination therapy, which included chemotherapy drugs, were evaluated for response. One patient achieved complete response, seven partial response and one remained with stable disease. Among these nine patients that continued into Phase II, five patients showed a 10% increase in activated CD8 + cells in their blood count. Despite a favourable outcome, this combination therapy did not achieve the pre-set threshold of a 20% objective response rate [154].
Navoximod/GDC-0919
Navoximod/GDC-0919 is an IDO1 inhibitor with selective inhibition against TDO [155,156,157]. Two clinical trials, NCT02048709 and NCT02471846, examined the potential of navoximod in the treatment of BrCa patients.
NCT02048709 is a Phase I trial that evaluated the safety profile of navoximod in patients with recurrent advanced solid tumours [158]. Out of the 22 patients enroled, only one patient was identified with BrCa and had received 28-day cycles of Navoximod 600 mg. The BrCa patient was later withdrawn from the trial based on the physician decision. This trial reported a dose dependent inhibition of IDO1 activity based on the concentration of plasma KYN. Navoximod was well tolerated at the maximal dosage of 800 mg BID or 600 mg on continuous dosing, and no maximal toxicity dosage was reached. However, this trial also reported that navoximod showed minimal anti-tumour activity.
NCT02471846 is a Phase I trial that investigated the safety and efficacy of navoximod in combination with the PD-L1 inhibitor, atezolizumab, in patients with locally advanced or metastatic solid tumours [159]. A total of 66 patients were enroled for the multiple ascending dose study of navoximod that included 13 patients with TNBC. The maximum toxicity dosage was not reached; the measurement of plasma KYN in these patients showed that the inhibition of IDO1 is dose dependent. However, none of the TNBC patients achieved any anti-tumour response. An expansion of the dose escalation cohort (dose expansion cohort) was conducted to recruit another 92 patients. Twelve enroled patients were TNBC. Only one patient from the total BrCa group achieved objective anti-tumour activity. A series of tumour biopsies was collected from the dose escalation and expansion cohorts pre- and on-treatment, and IHC of IDO1 was performed. IDO1 was reported to be upregulated in the tumour cells but not significantly in immune cells. Expression of IDO1 was not dose dependent and there was no significant increase in the CD8+ or CD4+ effector T cells or tumour-infiltrating leucocytes. Although the combination treatment safety profile indicated that it is tolerable, it did not provide significant improvement to patient outcome as compared to single atezoluzumab therapy.
Conclusion
Technologic progresses in early diagnostic have significantly reduced BrCa mortality rate through early cancer detection. Moreover, research has revealed the multiple underlying mechanisms of BrCa pathology, leading to the development of various targeted treatments aiming to improve BrCa patient outcomes further. However, treatment for advanced BrCa and/or metastasis remains a challenge. Immunotherapy has been one of the treatments to limit the spread of cancer, and the KP has emerged as a key mechanism for targeted treatments.
In this review, we have presented strong evidence that the KP is dysregulated in BrCa and that several of its enzymes may be potential targets to enhance the anti-tumoral immune response. In particular, the positive outcomes from animal studies have led to multiple clinical trials examining the potential of combining IDO1 inhibitor in combination treatments for cancer patients, including six clinical trials with BrCa patients. However, these combined therapies did not improve patient outcomes as compared to monotherapy. These discouraging results echoed the failed phase I/II clinical trial evaluating epacadostat plus pembrolizumab in malignant melanoma. There were no significant differences between the treatment groups for progression free survival in epacadostat and pembrolizumab arm (n = 354) or placebo plus pembrolizumab (n = 352) (4.7 months and 4.9 months respectively). In addition to this, treatment-related adverse events were reported in 10% of patients receiving the combination treatment and 9% of patients receiving the placebo [160]. It is noteworthy to mention that this may be due to the absence of an IDO1 status assessment of the tumour prior to patient enrolment as highlighted in a review by Van den Eynde et al. [161]. In the six trials with BrCa patients, only half of the six trials assessed IDO1 status of the tumour during the trial. Therefore, most of the patients who did not respond may not have tumour with IDO1 expression, thus skewing the clinical trial data [162].
There are also some concerns about IDO1 inhibition that would need to be addressed in future studies. The first research question to address is whether a compensatory mechanism is activated when IDO1 is inhibited. Considering that TDO and IDO2 are in the same node of the pathway, inhibition of IDO1 may lead to a feedback mechanism to overexpress TDO and/or IDO2. TDO may likely be the prime substitute enzyme as it is not only expressed constitutively [76, 163] but has also been suggested to replace IDO1 as the driver for the KP during metastases in a tumour metastasis mouse model study [164]. The other concern that requires attention is that IDO1 inhibitor may activate AhR due to its chemical structure [165]. Overactivation of AhR is common in cancer and is associated with poor prognosis [166]. Although the AhR activation by IDO1 inhibitor is weak compared to its natural ligands, this might potentially be a contributing factor to the negative outcome of IDO1 inhibitor clinical trials.
Overall, the KP has been shown to have a strong association with BrCa and may play a role in the pathology of this cancer. An in-depth understanding of the pathway could either lead to better development of KP inhibitors or a monitoring tool for treatment response for BrCa. As demonstrated in a recent publication of a clinical trial examining the combination of Indoximod with checkpoint inhibitors, the combination therapy was well tolerated and showed anti-tumour efficacy in patients with melanoma [167]. Ongoing research may likely show similar promise for BrCa patients.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Prim. 2019;5:66.
Ahmad A. Breast cancer statistics: recent trends. Adv Exp Med Biol. 2019;1152:1–7.
Yersal O, Barutca S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol. 2014;5:412–24.
Yeo SK, Guan JL. Breast cancer: multiple subtypes within a tumor? Trends Cancer. 2017;3:753–60.
Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–8.
Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23.
Li J, Chen Z, Su K, Zeng J. Clinicopathological classification and traditional prognostic indicators of breast cancer. Int J Clin Exp Pathol. 2015;8:8500–5.
Figueroa-Magalhaes MC, Jelovac D, Connolly R, Wolff AC. Treatment of HER2-positive breast cancer. Breast 2014;23:128–36.
Bergin ART, Loi S. Triple-negative breast cancer: recent treatment advances. F1000Res. 2019;8:1–11.
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50.
Johnson KS, Conant EF, Soo MS. Molecular subtypes of breast cancer: a review for breast radiologists. J Breast Imaging. 2020;3:12–24.
Pal S, Luchtenborg M, Davies EA, Jack RH. The treatment and survival of patients with triple negative breast cancer in a London population. Springerplus. 2014;3:553.
Ganesh K, Massague J. Targeting metastatic cancer. Nat Med. 2021;27:34–44.
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–21.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28.
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–7.
Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:397–404.
Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5:74–82.
Thomas R, Al-Khadairi G, Decock J. Immune checkpoint inhibitors in triple negative breast cancer treatment: promising future prospects. Front Oncol. 2020;10:600573.
Gouasmi R, Ferraro-Peyret C, Nancey S, Coste I, Renno T, Chaveroux C, et al. The kynurenine pathway and cancer: why keep it simple when you can make it complicated. Cancers (Basel). 2022;14:1–15.
Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–65.
Palego L, Betti L, Rossi A, Giannaccini G. Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J Amino Acids. 2016;2016:8952520.
Comai S, Bertazzo A, Brughera M, Crotti S. Tryptophan in health and disease. Adv Clin Chem. 2020;95:165–218.
Colabroy KL, Begley TP. Tryptophan catabolism: identification and characterization of a new degradative pathway. J Bacteriol. 2005;187:7866–9.
Ren S, Liu H, Licad E, Correia MA. Expression of rat liver tryptophan 2,3-dioxygenase in Escherichia coli: structural and functional characterization of the purified enzyme. Arch Biochem Biophys. 1996;333:96–102.
Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem. 1986;261:3648–53.
Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–13.
Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467–71.
Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids. 2013;45:1319–29.
Knox WE, Mehler AH. The conversion of tryptophan to kynurenine in liver. I. The coupled tryptophan peroxidase-oxidase system forming formylkynurenine. J Biol Chem. 1950;187:419–30.
Haber R, Bessette D, Hulihan-Giblin B, Durcan MJ, Goldman D. Identification of tryptophan 2,3-dioxygenase RNA in rodent brain. J Neurochem. 1993;60:1159–62.
Suzuki S, Tone S, Takikawa O, Kubo T, Kohno I, Minatogawa Y. Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J. 2001;355:425–9.
Minatogawa Y, Suzuki S, Ando Y, Tone S, Takikawa O. Tryptophan pyrrole ring cleavage enzymes in placenta. Adv Exp Med Biol. 2003;527:425–34.
Ren S, Correia MA. Heme: a regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch Biochem Biophys. 2000;377:195–203.
Badawy AA. Effects of pregnancy on tryptophan metabolism and disposition in the rat. Biochem J. 1988;255:369–72.
Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1178646917691938.
Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res. 2015;3:161–72.
Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–81.
Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–12.
Jones SP, Franco NF, Varney B, Sundaram G, Brown DA, de Bie J, et al. Expression of the kynurenine pathway in human peripheral blood mononuclear cells: implications for inflammatory and neurodegenerative disease. PLoS ONE. 2015;10:e0131389.
Robinson CM, Hale PT, Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res. 2005;25:20–30.
Guillemin GJ, Smythe GA, Veas LA, Takikawa O, Brew BJ. A beta 1-42 induces production of quinolinic acid by human macrophages and microglia. Neuroreport. 2003;14:2311–5.
Sono M, Taniguchi T, Watanabe Y, Hayaishi O. Indoleamine 2,3-dioxygenase. Equilibrium studies of the tryptophan binding to the ferric, ferrous, and CO-bound enzymes. J Biol Chem. 1980;255:1339–45.
Tanaka M, Vecsei L. Monitoring the kynurenine system: concentrations, ratios or what else? Adv Clin Exp Med. 2021;30:775–8.
Fukunaga M, Yamamoto Y, Kawasoe M, Arioka Y, Murakami Y, Hoshi M, et al. Studies on tissue and cellular distribution of indoleamine 2,3-dioxygenase 2: the absence of IDO1 upregulates IDO2 expression in the epididymis. J Histochem Cytochem. 2012;60:854–60.
Torok N, Tanaka M, Vecsei L. Searching for peripheral biomarkers in neurodegenerative diseases: the tryptophan-kynurenine metabolic pathway. Int J Mol Sci. 2020;21:1–24.
Beadle GW, Mitchell HK, Nyc JF. Kynurenine as an intermediate in the formation of nicotinic acid from tryptophane by neurospora. Proc Natl Acad Sci USA. 1947;33:155–8.
Wolf H. The effect of hormones and vitamin B6 on urinary excretion of metabolites of the kynurenine pathway. Scand J Clin Lab Invest Suppl. 1974;136:1–186.
Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem. 2009;109:316–25.
Phillips RS. Structure, mechanism, and substrate specificity of kynureninase. Biochim Biophys Acta. 2011;1814:1481–8.
Darlington LG, Forrest CM, Mackay GM, Smith RA, Smith AJ, Stoy N, et al. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res. 2010;3:51–9.
Alberati-Giani D, Cesura AM, Broger C, Warren WD, Rover S, Malherbe P. Cloning and functional expression of human kynurenine 3-monooxygenase. FEBS Lett. 1997;410:407–12.
Erickson JB, Flanagan EM, Russo S, Reinhard JF Jr. A radiometric assay for kynurenine 3-hydroxylase based on the release of 3H2O during hydroxylation of L-[3,5-3H]kynurenine. Anal Biochem. 1992;205:257–62.
Clement CC, D’Alessandro A, Thangaswamy S, Chalmers S, Furtado R, Spada S, et al. 3-hydroxy-L-kynurenamine is an immunomodulatory biogenic amine. Nat Commun. 2021;12:4447.
Fazio F, Lionetto L, Molinaro G, Bertrand HO, Acher F, Ngomba RT, et al. Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Mol Pharm. 2012;81:643–56.
Salter M, Knowles RG, Pogson CI. Quantification of the importance of individual steps in the control of aromatic amino acid metabolism. Biochem J. 1986;234:635–47.
Huang PC, Liu DK, Kao MS. The metabolism of alpha-N-monomethyl-L-tryptophan (L-abrin) in man and in normal and pyridoxine deficient rats. Tsa Chih Gaoxiong Yi Xue Yuan Tong Xue Hui. 1964;63:285–93.
Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68.
Routy JP, Routy B, Graziani GM, Mehraj V. The kynurenine pathway is a double-edged sword in immune-privileged sites and in cancer: implications for immunotherapy. Int J Tryptophan Res. 2016;9:67–77.
Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71.
Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–42.
Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. 2010;107:19961–6.
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8.
Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–90.
Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–54.
Lanis JM, Alexeev EE, Curtis VF, Kitzenberg DA, Kao DJ, Battista KD, et al. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 2017;10:1133–44.
Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207.
Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72:5435–40.
Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–9.
Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–900.
Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72.
Adams S, Teo C, McDonald KL, Zinger A, Bustamante S, Lim CK, et al. Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS ONE. 2014;9:e112945.
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203.
Chen IC, Lee KH, Hsu YH, Wang WR, Chen CM, Cheng YW. Expression pattern and clinicopathological relevance of the indoleamine 2,3-dioxygenase 1/tryptophan 2,3-dioxygenase protein in colorectal cancer. Dis Markers. 2016;2016:8169724.
Zhao L, Wang B, Yang C, Lin Y, Zhang Z, Wang S, et al. TDO2 knockdown inhibits colorectal cancer progression via TDO2-KYNU-AhR pathway. Gene. 2021;792:145736.
Heng B, Bilgin AA, Lovejoy DB, Tan VX, Milioli HH, Gluch L, et al. Differential kynurenine pathway metabolism in highly metastatic aggressive breast cancer subtypes: beyond IDO1-induced immunosuppression. Breast Cancer Res. 2020;22:113.
Liu Q, Zhai J, Kong X, Wang X, Wang Z, Fang Y, et al. Comprehensive analysis of the expression and prognosis for TDO2 in breast cancer. Mol Ther Oncol. 2020;17:153–68.
Li L, Wang T, Li S, Chen Z, Wu J, Cao W, et al. TDO2 promotes the EMT of hepatocellular carcinoma through Kyn-AhR pathway. Front Oncol. 2020;10:562823.
D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, et al. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75:4651–64.
Herrera-Rios D, Mughal SS, Teuber-Hanselmann S, Pierscianek D, Sucker A, Jansen P, et al. Macrophages/microglia represent the major source of indolamine 2,3-dioxygenase expression in melanoma metastases of the brain. Front Immunol. 2020;11:120.
Hsu YL, Hung JY, Chiang SY, Jian SF, Wu CY, Lin YS, et al. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7:27584–98.
Mansfield AS, Heikkila PS, Vaara AT, von Smitten KA, Vakkila JM, Leidenius MH. Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer. 2009;9:231.
Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77.
Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci USA. 2007;104:18619–24.
Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra6.
Zaher SS, Germain C, Fu H, Larkin DF, George AJ. 3-hydroxykynurenine suppresses CD4+ T-cell proliferation, induces T-regulatory-cell development, and prolongs corneal allograft survival. Invest Ophthalmol Vis Sci. 2011;52:2640–8.
Sahm F, Oezen I, Opitz CA, Radlwimmer B, von Deimling A, Ahrendt T, et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res. 2013;73:3225–34.
Leclerc D, Staats Pires AC, Guillemin GJ, Gilot D. Detrimental activation of AhR pathway in cancer: an overview of therapeutic strategies. Curr Opin Immunol. 2021;70:15–26.
Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, et al. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS ONE. 2014;9:e87877.
Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–51.
DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97.
Sadik A, Somarribas Patterson LF, Ozturk S, Mohapatra SR, Panitz V, Secker PF, et al. IL4I1 Is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell. 2020;182:1252–70.e34.
Rose DP. Tryptophan metabolism in carcinoma of the breast. Lancet. 1967;1:239–41.
Bell ED. Tryptophan metabolism in Japanese and British women and its relationship to endogenous steroid levels. Clin Chim Acta. 1978;85:33–40.
Davis HL Jr, Brown RR, Leklem J, Carlson IH. Tryptophan metabolism in breast cancer. Correlation with urinary steroid excretion. Cancer. 1973;31:1061–4.
Rose DP, Randall ZC. Tryptophan metabolism in early and advanced breast cancer and carcinoma of the cervix. Clin Chim Acta. 1972;40:276–80.
Sadok I, Rachwal K, Jonik I, Staniszewska M. Reliable chromatographic assay for measuring of indoleamine 2,3-dioxygenase 1 (IDO1) activity in human cancer cells. J Enzym Inhib Med Chem. 2021;36:581–92.
Zhai L, Ladomersky E, Lenzen A, Nguyen B, Patel R, Lauing KL, et al. IDO1 in cancer: a Gemini of immune checkpoints. Cell Mol Immunol. 2018;15:447–57.
Wei JL, Wu SY, Yang YS, Xiao Y, Jin X, Xu XE, et al. GCH1 induces immunosuppression through metabolic reprogramming and IDO1 upregulation in triple-negative breast cancer. J Immunother Cancer. 2021;9:1–14.
Carvajal-Hausdorf DE, Mani N, Velcheti V, Schalper KA, Rimm DL. Objective measurement and clinical significance of IDO1 protein in hormone receptor-positive breast cancer. J Immunother Cancer. 2017;5:81.
Dewi DL, Mohapatra SR, Blanco Cabanes S, Adam I, Somarribas Patterson LF, Berdel B, et al. Suppression of indoleamine-2,3-dioxygenase 1 expression by promoter hypermethylation in ER-positive breast cancer. Oncoimmunology. 2017;6:e1274477.
Sakurai K, Fujisaki S, Nagashima S, Maeda T, Shibata M, Gonda K, et al. [Analysis of indoleamine 2, 3-dioxygenase expression in breast cancer patients with bone metastasis]. Gan Kagaku Ryoho. 2012;39:1776–8.
Sakurai K, Fujisaki S, Suzuki S, Nagashima S, Maeda T, Tomita R, et al. [Indoleamine 2,3-dioxygenase activity in breast cancer patients with local recurrence or distant metastases]. Gan Kagaku Ryoho. 2014;41:1304–6.
Hufner K, Oberguggenberger A, Kohl C, Geisler S, Gamper E, Meraner V, et al. Levels in neurotransmitter precursor amino acids correlate with mental health in patients with breast cancer. Psychoneuroendocrinology. 2015;60:28–38.
Lyon DE, Walter JM, Starkweather AR, Schubert CM, McCain NL. Tryptophan degradation in women with breast cancer: a pilot study. BMC Res Notes. 2011;4:156.
Juhasz C, Nahleh Z, Zitron I, Chugani DC, Janabi MZ, Bandyopadhyay S, et al. Tryptophan metabolism in breast cancers: molecular imaging and immunohistochemistry studies. Nucl Med Biol. 2012;39:926–32.
Zhao Y, Wei L, Liu J, Li F. Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer. Cancer Chemother Pharm. 2020;85:77–93.
Tang X, Lin CC, Spasojevic I, Iversen ES, Chi JT, Marks JR. A joint analysis of metabolomics and genetics of breast cancer. Breast Cancer Res. 2014;16:415.
Chen JY, Li CF, Kuo CC, Tsai KK, Hou MF, Hung WC. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2,3-dioxygenase promotes breast cancer progression. Breast Cancer Res. 2014;16:410.
Wei L, Zhu S, Li M, Li F, Wei F, Liu J, et al. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front Immunol. 2018;9:724.
Onesti CE, Boemer F, Josse C, Leduc S, Bours V, Jerusalem G. Tryptophan catabolism increases in breast cancer patients compared to healthy controls without affecting the cancer outcome or response to chemotherapy. J Transl Med. 2019;17:239.
Isla Larrain MT, Rabassa ME, Lacunza E, Barbera A, Creton A, Segal-Eiras A, et al. IDO is highly expressed in breast cancer and breast cancer-derived circulating microvesicles and associated to aggressive types of tumors by in silico analysis. Tumour Biol. 2014;35:6511–9.
Ghafouri-Fard S, Taherian-Esfahani Z, Dashti S, Kholghi Oskooei V, Taheri M, Samsami M. Gene expression of indoleamine and tryptophan dioxygenases and three long non-coding RNAs in breast cancer. Exp Mol Pathol. 2020;114:104415.
Sakurai K, Fujisaki S, Adachi K, Suzuki S, Masuo Y, Nagashima S, et al. [Indoleamine 2,3-dioxygenase activity during fulvestrant therapy for multiple metastatic breast cancer patients]. Gan Kagaku Ryoho. 2016;43:1233–6.
Sakurai K, Fujisaki S, Nagashima S, Maeda T, Tomita R, Suzuki S, et al. [Indoleamine 2, 3-dioxygenase activity during neoadjuvant chemotherapy in patients with breast cancer]. Gan Kagaku Ryoho. 2013;40:1578–80.
Sakurai K, Fujisaki S, Nagashima S, Maeda T, Shibata M, Gonda K, et al. [Indoleamine 2, 3-dioxygenase activity during chemotherapy or trastuzumab therapy in patients with breast cancer]. Gan Kagaku Ryoho. 2012;39:1791–3.
Suzuki Y, Sakurai K, Adachi K, Kubota H, Suzuki S, Takei S, et al. [Indoleamine 2,3-Dioxygenase Activity during Long-Term Letrozole Therapy for Hormone Receptor-Positive Breast Cancer]. Gan To Kagaku Ryoho. 2018;45:1495–7.
Sakurai K, Fujisaki S, Kubota H, Hara Y, Suzuki S, Adachi K, et al. [Indoleamine 2,3-dioxygenase activity during letrozol therapy for an elderly breast cancer patient]. Gan Kagaku Ryoho. 2017;44:892–5.
Gao J, Deng F, Jia W. Inhibition of indoleamine 2,3-dioxygenase enhances the therapeutic efficacy of immunogenic chemotherapeutics in breast cancer. J Breast Cancer. 2019;22:196–209.
Cheong JE, Ekkati A, Sun L. A patent review of IDO1 inhibitors for cancer. Expert Opin Ther Pat. 2018;28:317–30.
Li Y, Gong S, Pan W, Chen Y, Liu B, Li N, et al. A tumor acidity activatable and Ca(2+)-assisted immuno-nanoagent enhances breast cancer therapy and suppresses cancer recurrence. Chem Sci. 2020;11:7429–37.
Ebokaiwe AP, Njoya EM, Sheng Y, Zhang Z, Li S, Zhou Z, et al. Salinomycin promotes T-cell proliferation by inhibiting the expression and enzymatic activity of immunosuppressive indoleamine-2,3-dioxygenase in human breast cancer cells. Toxicol Appl Pharm. 2020;404:115203.
Lan Y, Liang Q, Sun Y, Cao A, Liu L, Yu S, et al. Codelivered chemotherapeutic doxorubicin via a dual-functional immunostimulatory polymeric prodrug for breast cancer immunochemotherapy. ACS Appl Mater Interfaces. 2020;12:31904–21.
Kotecki N, Vuagnat P, O’Neil BH, Jalal S, Rottey S, Prenen H, et al. A phase I study of an IDO-1 Inhibitor (LY3381916) as monotherapy and in combination with an anti-PD-L1 antibody (LY3300054) in patients with advanced cancer. J Immunother. 2021;44:264–75.
Travers MT, Gow IF, Barber MC, Thomson J, Shennan DB. Indoleamine 2,3-dioxygenase activity and L-tryptophan transport in human breast cancer cells. Biochim Biophys Acta. 2004;1661:106–12.
Hill M, Pereira V, Chauveau C, Zagani R, Remy S, Tesson L, et al. Heme oxygenase-1 inhibits rat and human breast cancer cell proliferation: mutual cross inhibition with indoleamine 2,3-dioxygenase. FASEB J. 2005;19:1957–68.
Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharm Toxicol. 1997;37:517–54.
Lyon DE, Starkweather A, Yao Y, Garrett T, Kelly DL, Menzies V, et al. Pilot study of metabolomics and psychoneurological symptoms in women with early stage breast cancer. Biol Res Nurs. 2018;20:227–36.
Sakurai K, Fujisaki S, Nagashima S, Maeda T, Tomita R, Suzuki S, et al. [Indoleamine 2, 3-dioxygenase activity for breast cancer patients with recurrence 5 or more years after surgery]. Gan Kagaku Ryoho. 2013;40:1590–2.
Sakurai K, Fujisaki S, Nagashima S, Shibata M, Maeda T, Ueda Y, et al. [Long-term follow-up study: indolemamine 2,3-dioxygenase activity during chemotherapy or hormone therapy in patients with breast cancer]. Gan To Kagaku Ryoho. 2011;38:1930–2.
Kubota H, Sakurai K, Fujisaki S, Hara Y, Suzuki S, Adachi K, et al. [Clinical evaluation of indoleamine 2, 3-dioxygenase in the serum of patients with locally advanced breast cancer during mohs paste treatment]. Gan Kagaku Ryoho. 2017;44:915–7.
Sakurai K, Enomoto K, Kitajima A, Tani M, Amano S, Shiono M. [Indoleamine 2,3-dioxygenase expression in breast cancer patients during chemotherapy]. Gan Kagaku Ryoho. 2008;35:2265–7.
Greene LI, Bruno TC, Christenson JL, D’Alessandro A, Culp-Hill R, Torkko K, et al. A role for tryptophan-2,3-dioxygenase in CD8 T-cell suppression and evidence of tryptophan catabolism in breast cancer patient plasma. Mol Cancer Res. 2019;17:131–9.
Rogers TJ, Christenson JL, Greene LI, O’Neill KI, Williams MM, Gordon MA, et al. Reversal of triple-negative breast cancer EMT by miR-200c decreases tryptophan catabolism and a program of immunosuppression. Mol Cancer Res. 2019;17:30–41.
Novikov O, Wang Z, Stanford EA, Parks AJ, Ramirez-Cardenas A, Landesman E, et al. An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER-/PR-/Her2- human breast cancer cells. Mol Pharm. 2016;90:674–88.
Vacher S, Castagnet P, Chemlali W, Lallemand F, Meseure D, Pocard M, et al. High AHR expression in breast tumors correlates with expression of genes from several signaling pathways namely inflammation and endogenous tryptophan metabolism. PLoS ONE. 2018;13:e0190619.
Bekki K, Vogel H, Li W, Ito T, Sweeney C, Haarmann-Stemmann T, et al. The aryl hydrocarbon receptor (AhR) mediates resistance to apoptosis induced in breast cancer cells. Pestic Biochem Physiol. 2015;120:5–13.
Huang TT, Tseng LM, Chen JL, Chu PY, Lee CH, Huang CT, et al. Kynurenine 3-monooxygenase upregulates pluripotent genes through beta-catenin and promotes triple-negative breast cancer progression. EBioMedicine. 2020;54:102717.
Lai MH, Liao CH, Tsai NM, Chang KF, Liu CC, Chiu YH, et al. Surface expression of kynurenine 3-monooxygenase promotes proliferation and metastasis in triple-negative breast cancers. Cancer Control. 2021;28:10732748211009245.
Tsang YW, Liao CH, Ke CH, Tu CW, Lin CS. Integrated molecular characterization to reveal the association between kynurenine 3-monooxygenase expression and tumorigenesis in human breast cancers. J Pers Med. 2021;11:1–14.
Pires AS, Sundaram G, Heng B, Krishnamurthy S, Brew BJ, Guillemin GJ. Recent advances in clinical trials targeting the kynurenine pathway. Pharm Ther. 2021;236:108055.
Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ, et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther. 2010;9:489–98.
Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–30.
Jochems C, Fantini M, Fernando RI, Kwilas AR, Donahue RN, Lepone LM, et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific T cells. Oncotarget. 2016;7:37762–72.
Mitchell TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Olszanski AJ, et al. Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase I results from a multicenter, open-label phase I/II trial (ECHO-202/KEYNOTE-037). J Clin Oncol. 2018;36:3223–30.
Hamid O, Bauer TM, Spira AI, Smith DC, Olszanski AJ, Tarhini AA, et al. Safety of epacadostat 100 mg bid plus pembrolizumab 200 mg Q3W in advanced solid tumors: Phase 2 data from ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35:3012.
Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460–8.
Brincks EL, Adams J, Wang L, Turner B, Marcinowicz A, Ke J, et al. Indoximod opposes the immunosuppressive effects mediated by IDO and TDO via modulation of AhR function and activation of mTORC1. Oncotarget. 2020;11:2438–61.
Valvezan AJ, Manning BD. Molecular logic of mTORC1 signalling as a metabolic rheostat. Nat Metab. 2019;1:321–33.
Mariotti V, Han H, Ismail-Khan R, Tang SC, Dillon P, Montero AJ, et al. Effect of taxane chemotherapy with or without indoximod in metastatic breast cancer: a randomized clinical trial. JAMA Oncol. 2021;7:61–9.
Soliman H, Khambati F, Han HS, Ismail-Khan R, Bui MM, Sullivan DM, et al. A phase-1/2 study of adenovirus-p53 transduced dendritic cell vaccine in combination with indoximod in metastatic solid tumors and invasive breast cancer. Oncotarget. 2018;9:10110–7.
Mautino MR, Jaipuri FA, Waldo J, Kumar S, Adams J, Van Allen C, et al. Abstract 491: NLG919, a novel indoleamine-2,3-dioxygenase (IDO)-pathway inhibitor drug candidate for cancer therapy. Cancer Res. 2013;73:491.
Mautino MR, Link CJ, Vahanian NN, Adams JT, Allen CV, Sharma MD, et al. Abstract 5023: Synergistic antitumor effects of combinatorial immune checkpoint inhibition with anti-PD-1/PD-L antibodies and the IDO pathway inhibitors NLG-919 and indoximod in the context of active immunotherapy. Cancer Res. 2014;74:5023.
Spahn J, Peng J, Lorenzana E, Kan D, Hunsaker T, Segal E, et al. Improved anti-tumor immunity and efficacy upon combination of the IDO1 inhibitor GDC-0919 with anti-PD-l1 blockade versus anti-PD-l1 alone in preclinical tumor models. J Immunother Cancer. 2015;3:P303.
Nayak-Kapoor A, Hao Z, Sadek R, Dobbins R, Marshall L, Vahanian NN, et al. Phase Ia study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent advanced solid tumors. J Immunother Cancer. 2018;6:61.
Jung KH, LoRusso P, Burris H, Gordon M, Bang YJ, Hellmann MD, et al. Phase I study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) administered with PD-L1 inhibitor (atezolizumab) in advanced solid tumors. Clin Cancer Res. 2019;25:3220–8.
Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–97.
Eynde BJVD, Baren NV, Baurain J-F. Is there a clinical future for IDO1 inhibitors after the failure of epacadostat in melanoma? Annu Rev Cancer Biol. 2020;4:241–56.
Fujiwara Y, Kato S, Nesline MK, Conroy JM, DePietro P, Pabla S, et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 2022;110:102461.
Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA. 2012;109:2497–502.
Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–35.
Moyer BJ, Rojas IY, Murray IA, Lee S, Hazlett HF, Perdew GH, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors activate the aryl hydrocarbon receptor. Toxicol Appl Pharm. 2017;323:74–80.
Xue P, Fu J, Zhou Y. The aryl hydrocarbon receptor and tumor immunity. Front Immunol. 2018;9:286.
Zakharia Y, McWilliams RR, Rixe O, Drabick J, Shaheen MF, Grossmann KF, et al. Phase II trial of the IDO pathway inhibitor indoximod plus pembrolizumab for the treatment of patients with advanced melanoma. J Immunother Cancer. 2021;9:1–9.
Girgin G, Tolga Sahin T, Fuchs D, Kasuya H, Yuksel O, Tekin E, et al. Immune system modulation in patients with malignant and benign breast disorders: tryptophan degradation and serum neopterin. Int J Biol Markers. 2009;24:265–70.
Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer AM, et al. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. 2013;34:108–19.
Puccetti P, Fallarino F, Italiano A, Soubeyran I, MacGrogan G, Debled M, et al. Accumulation of an endogenous tryptophan-derived metabolite in colorectal and breast cancers. PLoS ONE. 2015;10:e0122046.
Sakurai K, Fujisaki S, Kubota H, Hara Y, Suzuki S, Adachi K, et al. [Indoleamine 2,3-dioxygenase activity during fulvestrant therapy for aromatase inhibitor resistant metastatic breast cancer]. Gan Kagaku Ryoho. 2017;44:886–8.
Xin Y, Cai H. Improved radiosynthesis and biological evaluations of L- and D-1-[(18)F]fluoroethyl-tryptophan for PET imaging of IDO-mediated kynurenine pathway of tryptophan metabolism. Mol Imaging Biol. 2017;19:589–98.
Madssen TS, Thune I, Flote VG, Lundgren S, Bertheussen GF, Frydenberg H, et al. Metabolite and lipoprotein responses and prediction of weight gain during breast cancer treatment. Br J Cancer. 2018;119:1144–54.
Triplett TA, Garrison KC, Marshall N, Donkor M, Blazeck J, Lamb C, et al. Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol. 2018;36:758–64.
Zimmer P, Schmidt ME, Prentzell MT, Berdel B, Wiskemann J, Kellner KH, et al. Resistance exercise reduces kynurenine pathway metabolites in breast cancer patients undergoing radiotherapy. Front Oncol. 2019;9:962.
Liu CL, Cheng SP, Chen MJ, Lin CH, Chen SN, Kuo YH, et al. Quinolinate phosphoribosyltransferase promotes invasiveness of breast cancer through myosin light chain phosphorylation. Front Endocrinol (Lausanne). 2020;11:621944.
Sadok I, Tyszczuk-Rotko K, Mroczka R, Staniszewska M. Simultaneous voltammetric analysis of tryptophan and kynurenine in culture medium from human cancer cells. Talanta. 2020;209:120574.
Saito N, Kanno Y, Yamashita N, Degawa M, Yoshinari K, Nemoto K. The differential selectivity of aryl hydrocarbon receptor (AHR) agonists towards AHR-dependent suppression of mammosphere formation and gene transcription in human breast cancer cells. Biol Pharm Bull. 2021;44:571–8.
Perez-Tejada J, Labaka A, Vegas O, Larraioz A, Pescador A, Arregi A. Anxiety and depression after breast cancer: The predictive role of monoamine levels. Eur J Oncol Nurs. 2021;52:101953.
Acknowledgements
All figures were created using Biorender.com.
Funding
HNG is supported by Macquarie University Research Excellence Scholarship—Master of Research scholarship; SBA is supported by Cancer Council NSW funding RG19-04; GJG is supported by a Fellowship from the National Health and Medical Research Council (NHMRC) APP1176660, and Macquarie University; BH is supported by NHMRC. Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Conceptualisation: HNG, ASP, LG and BH; writing—original draft preparation, review and editing: HNG, ASP, GJG, LG, SBA and BH.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Girithar, HN., Staats Pires, A., Ahn, S.B. et al. Involvement of the kynurenine pathway in breast cancer: updates on clinical research and trials. Br J Cancer 129, 185–203 (2023). https://doi.org/10.1038/s41416-023-02245-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02245-7
This article is cited by
-
Metabolic reprogramming in the tumor microenvironment of liver cancer
Journal of Hematology & Oncology (2024)