Abstract
Background
Hyperglycaemia is a well-known initial symptom in patients with pancreatic ductal adenocarcinoma (PDAC). Metabolic reprogramming in cancer, described as the Warburg effect, can induce epithelial-mesenchymal transition (EMT).
Methods
The biological impact of hyperglycaemia on malignant behaviour in PDAC was examined by in vitro and in vivo experiments.
Results
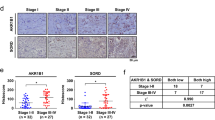
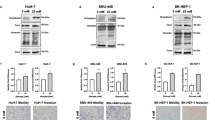
Hyperglycaemia promoted EMT by inducing metabolic reprogramming into a glycolytic phenotype via yes-associated protein (YAP)/PDZ-binding motif (TAZ) overexpression, accompanied by GLUT1 overexpression and enhanced phosphorylation Akt in PDAC. In addition, hyperglycaemia enhanced chemoresistance by upregulating ABCB1 expression and triggered PDAC switch into pure basal-like subtype with activated Hedgehog pathway (GLI1 high, GATA6 low expression) through YAP/TAZ overexpression. PDAC is characterised by abundant stroma that harbours tumour-promoting properties and chemoresistance. Hyperglycaemia promotes the production of collagen fibre-related proteins (fibronectin, fibroblast activation protein, COL1A1 and COL11A1) by stimulating YAP/TAZ expression in cancer-associated fibroblasts (CAFs). Knockdown of YAP and/or TAZ or treatment with YAP/TAZ inhibitor (K975) abolished EMT, chemoresistance and a favourable tumour microenvironment even under hyperglycemic conditions in vitro and in vivo.
Conclusion
Hyperglycaemia induces metabolic reprogramming into glycolytic phenotype and promotes EMT via YAP/TAZ-Hedgehog signalling axis, and YAP/TAZ could be a novel therapeutic target in PDAC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All presented data are available from the corresponding author upon reasonable request.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas. 2008;36:e15–20.
Oldfield LE, Connor AA, Gallinger S. Molecular events in the natural history of pancreatic cancer. Trends Cancer. 2017;3:336–46.
Norris AL, Roberts NJ, Jones S, Wheelan SJ, Papadoupoulous N, Vogelstein B, et al. Familial and sporadic pancreatic cancer share the same molecular pathogenesis. Fam Cancer. 2015;14:95–103.
Li S, Zhu H, Chen H, Xia J, Zhang F, Xu R, et al. Glucose promotes epithelial-mesenchymal transitions in bladder cancer by regulating the functions of YAP1 and TAZ. J Cell Mol Med. 2020;24:10391–401.
Li M, Bu X, Cai B, Liang P, Li K, Qu X, et al. Biological role of metabolic reprogramming of cancer cells during epithelialmesenchymal transition (Review). Oncol Rep. 2019;41:727–41.
Maugeri-Sacca M, De Maria R. The Hippo pathway in normal development and cancer. Pharm Ther. 2018;186:60–72.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61.
Koo JH, Plouffe SW, Meng Z, Lee DH, Yang D, Lim DS, et al. Induction of AP-1 by YAP/TAZ contributes to cell proliferation and organ growth. Genes Dev. 2020;34:72–86.
Yasuda T, Koiwa M, Yonemura A, Akiyama T, Baba H, Ishimoto T. Protocol to establish cancer-associated fibroblasts from surgically resected tissues and generate senescent fibroblasts. STAR Protoc. 2021;2:100553.
Mima K, Okabe H, Ishimoto T, Hayashi H, Nakagawa S, Kuroki H, et al. CD44s regulates the TGF-beta-mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. 2012;72:3414–23.
Hayashi H, Higashi T, Yokoyama N, Kaida T, Sakamoto K, Fukushima Y, et al. An imbalance in TAZ and YAP expression in hepatocellular carcinoma confers cancer stem cell-like behaviors contributing to disease progression. Cancer Res. 2015;75:4985–97.
Kosumi K, Baba Y, Sakamoto A, Ishimoto T, Harada K, Nakamura K, et al. Lysine-specific demethylase-1 contributes to malignant behavior by regulation of invasive activity and metabolic shift in esophageal cancer. Int J Cancer. 2016;138:428–39.
Hayashi H, Beppu T, Sakamoto Y, Miyamoto Y, Yokoyama N, Higashi T, et al. Prognostic value of Ki-67 expression in conversion therapy for colorectal liver-limited metastases. Am J Cancer Res. 2015;5:1225–33.
Chikamoto A, Inoue R, Komohara Y, Sakamaki K, Hashimoto D, Shiraishi S, et al. Preoperative high maximum standardized uptake value in association with glucose transporter 1 predicts poor prognosis in pancreatic cancer. Ann Surg Oncol. 2017;24:2040–6.
Hayashi H, Sakai K, Baba H, Sakai T. Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice. Hepatology. 2012;55:1562–73.
Qin C, Yang G, Yang J, Ren B, Wang H, Chen G, et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. 2020;19:50.
Li J, Wu H, Li W, Yin L, Guo S, Xu X, et al. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-kappaB signaling. Oncogene. 2016;35:5501–14.
Moitra K. Overcoming multidrug resistance in cancer stem cells. Biomed Res Int. 2015;2015:635745.
Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology. 2018;155:1999–2013.e1993.
Kozhemyakina E, Ionescu A, Lassar AB. GATA6 is a crucial regulator of Shh in the limb bud. PLoS Genet. 2014;10:e1004072.
Steele NG, Biffi G, Kemp SB, Zhang Y, Drouillard D, Syu L, et al. Inhibition of Hedgehog signaling alters fibroblast composition in pancreatic cancer. Clin Cancer Res. 2021;27:2023–37.
Fujii M, Toyoda T, Nakanishi H, Yatabe Y, Sato A, Matsudaira Y, et al. TGF-beta synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J Exp Med. 2012;209:479–94.
Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–44.
Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–56.
Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–91.
Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–41.
Lin YT, Ding JY, Li MY, Yeh TS, Wang TW, Yu JY. YAP regulates neuronal differentiation through Sonic hedgehog signaling pathway. Exp Cell Res. 2012;318:1877–88.
Chen HJ, Wang CM, Wang TW, Liaw GJ, Hsu TH, Lin TH, et al. The Hippo pathway controls polar cell fate through Notch signaling during Drosophila oogenesis. Dev Biol. 2011;357:370–9.
Yu J, Poulton J, Huang YC, Deng WM. The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS ONE. 2008;3:e1761.
Rozengurt E, Eibl G. Crosstalk between KRAS, SRC and YAP signaling in pancreatic cancer: interactions leading to aggressive disease and drug resistance. Cancers (Basel). 2021;13:5126–46.
Koikawa K, Kibe S, Suizu F, Sekino N, Kim N, Manz TD, et al. Targeting Pin1 renders pancreatic cancer eradicable by synergizing with immunochemotherapy. Cell. 2021;184:4753–71.e4727.
Zhou XZ, Lu KP. The isomerase PIN1 controls numerous cancer-driving pathways and is a unique drug target. Nat Rev Cancer. 2016;16:463–78.
Amin S, Mhango G, Lin J, Aronson A, Wisnivesky J, Boffetta P, et al. Metformin improves survival in patients with pancreatic ductal adenocarcinoma and pre-existing diabetes: a propensity score analysis. Am J Gastroenterol. 2016;111:1350–7.
Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42.
Gruber R, Panayiotou R, Nye E, Spencer-Dene B, Stamp G, Behrens AYAP1. and TAZ control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterology. 2016;151:526–39.
Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37.
Halldorsson S, Rohatgi N, Magnusdottir M, Choudhary KS, Gudjonsson T, Knutsen E, et al. Metabolic re-wiring of isogenic breast epithelial cell lines following epithelial to mesenchymal transition. Cancer Lett. 2017;396:117–29.
Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H. Chemoresistance in pancreatic cancer. Int J Mol Sci. 2019;20:4504–22.
Gzil A, Zarębska I, Bursiewicz W, Antosik P, Grzanka D, Szylberg Ł. Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol Biol Rep. 2019;46:6629–45.
Lv Z, Guo Y. Metformin and its benefits for various diseases. Front Endocrinol (Lausanne). 2020;11:191.
Hayashi H, Higashi T, Miyata T, Yamashita YI, Baba H. Recent advances in precision medicine for pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2021;5:457–66.
O’Kane GM, Grünwald BT, Jang GH, Masoomian M, Picardo S, Grant RC, et al. GATA6 expression distinguishes classical and basal-like subtypes in advanced pancreatic cancer. Clin Cancer Res. 2020;26:4901–10.
Li Z, Sun C, Qin Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics. 2021;11:8322–36.
Srivastava SP, Goodwin JE. Cancer biology and prevention in diabetes. Cells. 2020;9:1380–1415.
Foster CT, Gualdrini F, Treisman R. Mutual dependence of the MRTF-SRF and YAP-TEAD pathways in cancer-associated fibroblasts is indirect and mediated by cytoskeletal dynamics. Genes Dev. 2017;31:2361–75.
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–46.
Acknowledgements
We thank Dr. Hiromitsu Hayashi, Dr. Norio Uemura and Dr. Kazuki Matsumura for helping with the study; Ms. Ogata, Ms. Yasuda and Ms. Taniguchi for their excellent technical assistance; Dr. Feng Wei, Dr. Chuan Lan and Dr. Xiyu Wu for giving me good advices; Shigeki Nakagawa, Kosuke Mima, Katsunori Imai, Yo-ichi Yamashita and Prof. Hideo Baba for assistance with revision of the paper. Data sharing requests will be considered by the management group upon written request to the corresponding author.
Funding
This work was supported by a Grant-in-Aid for Scientists (C); the Ministry of Education, Culture, Sports, Science, and Technology of Japan, No. 19K09177 (to HH); the Takeda Science Foundation, Japan (to HH); Japanese Foundation for Multidisciplinary Treatment of Cancer, Japan (to HH) and JST SPRING, Grant Number JPMJSP2127 (to ZL).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: ZL and HH. Performed the experiments: ZL, NU, KM and YO. Analysed the data: ZL and HH. Wrote the manuscript: HH and ZL. Collected clinical samples: ZL, NU, KM, HS, YS, TM and TH. Organised the paper and approved the final version to be published: HH, SN, KM, KI and HB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of Kumamoto University (Project No. 1291), and written informed consent was obtained from all human subjects.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent for publication Not applicable
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Hayashi, H., Matsumura, K. et al. Hyperglycaemia induces metabolic reprogramming into a glycolytic phenotype and promotes epithelial-mesenchymal transitions via YAP/TAZ-Hedgehog signalling axis in pancreatic cancer. Br J Cancer 128, 844–856 (2023). https://doi.org/10.1038/s41416-022-02106-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-02106-9
This article is cited by
-
GLUT1-mediated microglial proinflammatory activation contributes to the development of stress-induced spatial learning and memory dysfunction in mice
Cell & Bioscience (2024)
-
Identification of fibronectin type III domain containing 3B as a potential prognostic and therapeutic target for pancreatic cancer: a preliminary analysis
European Journal of Medical Research (2024)
-
miR-590-5p/Tiam1-mediated glucose metabolism promotes malignant evolution of pancreatic cancer by regulating SLC2A3 stability
Cancer Cell International (2023)