Abstract
Background
Human papillomavirus (HPV) testing on self-samples represents a great opportunity to increase cervical cancer screening uptake among under-screened women.
Methods
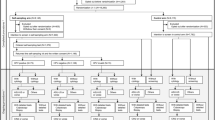
A systematic review and meta-analysis on randomised controlled trials (RCTs) were performed to update the evidence on the efficacy of strategies for offering self-sampling kits for HPV testing compared to conventional invitations and to compare different self-sampling invitation scenarios. Four experimental invitational scenarios were considered. Women in the control group were invited for screening according to existing practice: collection of a cervical specimen by a healthcare professional. Random-effects models were used to pool proportions, relative participation rates and absolute participation differences.
Results
Thirty-three trials were included. In the intention-to-treat analysis, all self-sampling invitation scenarios were more effective in reaching under-screened women compared to controls. Pooled participation difference (PD) and 95% confidence interval (CI) for experimental vs. control was 13.2% (95% CI = 11.0–15.3%) for mail-to-all, 4.4% (95% CI = 1.2–7.6%) for opt-in, 39.1% (95% CI = 8.4–69.9%) for community mobilisation & outreach and 28.1% (23.5–32.7%) for offer at healthcare service. PD for the comparison opt-in vs. mail-to-all, assessed in nine trials, was −8.2% (95% CI = −10.8 to −5.7%).
Discussion
Overall, screening participation was higher among women invited for self-sampling compared to control, regardless of the invitation strategy used. Opt-in strategies were less effective than send-to-all strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during this study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–203.
Catarino R, Petignat P, Dongui G, Vassilakos P. Cervical cancer screening in developing countries at a crossroad: emerging technologies and policy choices. World J Clin Oncol. 2015;6:281.
WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva, CH: World Health Organization; 2020.
Scarinci IC, Garcia FAR, Kobetz E, Partridge EE, Brandt HM, Bell MC, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116:2531–42.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Human papillomaviruses. Vol. 90. Lyon, FR: International Agency for Research on Cancer; 2007.
Arbyn M, Ronco G, Anttila A, Meijer CJLM, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30:F88–99.
Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJF, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32.
Arbyn M, Verdoodt F, Snijders PJF, Verhoef VMJ, Suonio E, Dillner L, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172–83.
Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
Arbyn M, Castle PE. Offering self-sampling kits for HPV Testing to reach women who do not attend in the regular cervical cancer screening program. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2015;24:769–72.
Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007;45:93–106.
Andrae B, Kemetli L, Sparén P, Silfverdal L, Strander B, Ryd W, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008;100:622–9.
Jalili F, O’Conaill C, Templeton K, Lotocki R, Fischer G, Manning L, et al. Assessing the impact of mailing self-sampling kits for human papillomavirus testing to unscreened non-responder women in Manitoba. Curr Oncol. 2019;26:167–72.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Harris R, Bradburn M, Deeks DJJ, Harbord HR, Altman ADG, Sterne J. metan: fixed- and random-effects meta-analysis. Stata J. 2008;8:3–28.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
StataCorp. Stata: Release 16. Statistical software. College Station, Texas, USA: College Station, Texas, USA: Stata Corp LLC; 2019.
Veerus P, Hallik R, Jänes J, Jõers K, Paapsi K, Laidra K, et al. Human papillomavirus self-sampling for long-term non-attenders in cervical cancer screening: a randomised feasibility study in Estonia. J Med Screen. 2022;29:53–60.
Bais AG, van Kemenade FJ, Berkhof J, Verheijen RHM, Snijders PJF, Voorhorst F, et al. Human papillomavirus testing on self-sampled cervicovaginal brushes: an effective alternative to protect nonresponders in cervical screening programs. Int J Cancer. 2007;120:1505–10.
Gök M, Heideman DAM, van Kemenade FJ, Berkhof J, Rozendaal L, Spruyt JWM, et al. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ. 2010;340:c1040.
Giorgi Rossi P, Marsili LM, Camilloni L, Iossa A, Lattanzi A, Sani C, et al. The effect of self-sampled HPV testing on participation to cervical cancer screening in Italy: a randomised controlled trial (ISRCTN96071600). Br J Cancer. 2011;104:248–54.
Piana L, Leandri FX, Le RL, Heid P, Tamalet C, Sancho-Garnier H. L’auto-prélèvement vaginal à domicile pour recherche de papilloma virus à haut risque. Une solution de remplacement pour les femmes ne participant pas au dépistage cytologique des cancers du col de l’utérus. Campagne expérimentale du département des Bouches-duRhône. Bull Cancer. 2011;98:723–31.
Szarewski A, Cadman L, Mesher D, Austin J, Ashdown-Barr L, Edwards R, et al. HPV self-sampling as an alternative strategy in non-attenders for cervical screening—a randomised controlled trial. Br J Cancer. 2011;104:915–20.
Virtanen A, Nieminen P, Luostarinen T, Anttila A. Self-sample HPV tests as an intervention for nonattendees of cervical cancer screening in Finland: a randomized trial. Cancer Epidemiol Biomark Prev. 2011;20:1960–9.
Wikström I, Lindell M, Sanner K, Wilander E. Self-sampling and HPV testing or ordinary Pap-smear in women not regularly attending screening: a randomised study. Br J Cancer. 2011;105:337–9.
Gök M, van Kemenade FJ, Heideman DAM, Berkhof J, Rozendaal L, Spruyt JWM, et al. Experience with high-risk human papillomavirus testing on vaginal brush-based self-samples of non-attendees of the cervical screening program. Int J Cancer. 2012;130:1128–35.
Darlin L, Borgfeldt C, Forslund O, Hénic E, Hortlund M, Dillner J, et al. Comparison of use of vaginal HPV self-sampling and offering flexible appointments as strategies to reach long-term non-attending women in organized cervical screening. J Clin Virol. 2013;58:155–60.
Sancho-Garnier H, Tamalet C, Halfon P, Leandri FX, Retraite LL, Djoufelkit K, et al. HPV self-sampling or the Pap-smear: a randomized study among cervical screening nonattenders from lower socioeconomic groups in France: HPV self-sampling or the Pap-smear for screening among nonattenders women? Int J Cancer. 2013;133:2681–7.
Haguenoer K, Sengchanh S, Gaudy-Graffin C, Boyard J, Fontenay R, Marret H, et al. Vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: a randomised trial. Br J Cancer. 2014;111:2187–96.
Cadman L, Wilkes S, Mansour D, Austin J, Ashdown-Barr L, Edwards R, et al. A randomized controlled trial in non-responders from Newcastle upon Tyne invited to return a self-sample for human papillomavirus testing versus repeat invitation for cervical screening. J Med Screen. 2015;22:28–37.
Giorgi Rossi P, Fortunato C, Barbarino P, Boveri S, Caroli S, Del Mistro A, et al. Self-sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. Br J Cancer. 2015;112:667–75.
Enerly E, Bonde J, Schee K, Pedersen H, Lönnberg S, Nygård M. Self-sampling for human papillomavirus testing among non-attenders increases attendance to the Norwegian cervical cancer screening programme. PLoS ONE. 2016;11:e0151978.
Racey CS, Gesink DC, Burchell AN, Trivers S, Wong T, Rebbapragada A. Randomized intervention of self-collected sampling for human papillomavirus testing in under-screened rural women: uptake of screening and acceptability. J Women’s Health. 2016;25:489–97.
Sultana F, English DR, Simpson JA, Drennan KT, Mullins R, Brotherton JML, et al. Home-based HPV self-sampling improves participation by never-screened and under-screened women: results from a large randomized trial (iPap) in Australia: home-based HPV self-sampling in never- and under-screened women. Int J Cancer. 2016;139:281–90.
Kitchener H, Gittins M, Cruickshank M, Moseley C, Fletcher S, Albrow R, et al. A cluster randomized trial of strategies to increase uptake amongst young women invited for their first cervical screen: the STRATEGIC trial. J Med Screen. 2018;25:88–98.
Ivanus U, Jerman T, Fokter AR, Takac I, Prevodnik VK, Marcec M, et al. Randomised trial of HPV self-sampling among non-attenders in the Slovenian cervical screening programme ZORA: comparing three different screening approaches. Radio Oncol. 2018;52:399–412.
Kellen E, Benoy I, Vanden Broeck D, Martens P, Bogers JP, Haelens A, et al. A randomized, controlled trial of two strategies of offering the home-based HPV self-sampling test to non-participants in the Flemish cervical cancer screening program: home-based HPV self-sampling test. Int J Cancer. 2018;143:861–8.
Tranberg M, Bech BH, Blaakær J, Jensen JS, Svanholm H, Andersen B. Preventing cervical cancer using HPV self-sampling: direct mailing of test-kits increases screening participation more than timely opt-in procedures—a randomized controlled trial. BMC Cancer. 2018;18:273.
Elfström KM, Sundström K, Andersson S, Bzhalava Z, Carlsten Thor A, Gzoul Z, et al. Increasing participation in cervical screening by targeting long-term nonattenders: randomized health services study. Int J Cancer. 2019;145:3033–9.
Winer RL, Lin J, Tiro JA, Miglioretti DL, Beatty T, Gao H, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729.
Lilliecreutz C, Karlsson H, Spetz, Holm AC. Participation in interventions and recommended follow-up for non-attendees in cervical cancer screening -taking the women’s own preferred test method into account—a Swedish randomised controlled trial. PLoS ONE. 2020;15:e0235202.
Brewer N, Bartholomew K, Grant J, Maxwell A, McPherson G, Wihongi H, et al. Acceptability of human papillomavirus (HPV) self-sampling among never- and under-screened Indigenous and other minority women: a randomised three-arm community trial in Aotearoa New Zealand. Lancet Reg Health West Pac. 2021;16:100265.
Broberg G, Gyrd-Hansen D, Miao Jonasson J, Ryd ML, Holtenman M, Milsom I, et al. Increasing participation in cervical cancer screening: offering a HPV self-test to long-term non-attendees as part of RACOMIP, a Swedish randomized controlled trial. Int J Cancer. 2014;134:2223–30.
Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, Salmerón J, Uribe P, Velasco-Mondragón E, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868–73.
Arrossi S, Thouyaret L, Herrero R, Campanera A, Magdaleno A, Cuberli M, et al. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob Health. 2015;3:e85–94.
Moses E, Pedersen HN, Mitchell SM, Sekikubo M, Mwesigwa D, Singer J, et al. Uptake of community-based, self-collected HPV testing vs. visual inspection with acetic acid for cervical cancer screening in Kampala, Uganda: preliminary results of a randomised controlled trial. Trop Med Int Health. 2015;20:1355–67.
Modibbo F, Iregbu KC, Okuma J, Leeman A, Kasius A, de Koning M, et al. Randomized trial evaluating self-sampling for HPV DNA based tests for cervical cancer screening in Nigeria. Infect Agent Cancer. 2017;12:11.
Gizaw M, Teka B, Ruddies F, Abebe T, Kaufmann AM, Worku A, et al. Uptake of cervical cancer screening in Ethiopia by self-sampling HPV DNA compared to visual inspection with acetic acid: a cluster randomized trial. Cancer Prev Res Philos Pa. 2019;12:609–16.
MacDonald EJ, Geller S, Sibanda N, Stevenson K, Denmead L, Adcock A, et al. Reaching under-screened/never-screened indigenous peoples with human papilloma virus self-testing: a community-based cluster randomised controlled trial. Aust N. Z J Obstet Gynaecol. 2021;61:135–41.
Verdoodt F, Jentschke M, Hillemanns P, Racey CS, Snijders PJF, Arbyn M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur J Cancer. 2015;51:2375–85.
Serrano B, Ibáñez R, Robles C, Peremiquel-Trillas P, de Sanjosé S, Bruni L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev Med. 2022;154:106900.
Elfström KM, Dillner J. Cervical cancer screening improvements with self-sampling during the COVID-19 pandemic. medRxiv. [Preprint] 2022. Available from: https://www.medrxiv.org/content/10.1101/2022.07.19.22277806v1.full.
Australian Government - Department of Health and Aged Care. Self collection to increase choice within the National Cervical Screening Program [Internet]. 2021 [cited 2022 Sep 28]. https://www.health.gov.au/news/self-collection-to-increase-choice-within-the-national-cervical-screening-program
Nishimura H, Yeh PT, Oguntade H, Kennedy CE, Narasimhan M. HPV self-sampling for cervical cancer screening: a systematic review of values and preferences. BMJ Glob Health. 2021;6:e003743.
McLachlan E, Anderson S, Hawkes D, Saville M, Arabena K. Completing the cervical screening pathway: factors that facilitate the increase of self-collection uptake among under-screened and never-screened women, an Australian pilot study. Curr Oncol. 2018;25:17–26.
Peeters E, Cornet K, Devroey D, Arbyn M. Efficacy of strategies to increase participation in cervical cancer screening: GPs offering self-sampling kits for HPV testing versus recommendations to have a pap smear taken—a randomised controlled trial. Papillomavirus Res. 2020;9:100194.
Aitken CA, Inturrisi F, Kaljouw S, Nieboer D, Siebers AG, Melchers WJG, et al. Sociodemographic characteristics and screening outcomes of women preferring self-sampling in the Dutch cervical cancer screening programme: a population-based study. Cancer Epidemiol Biomarkers Prev. 2022. https://doi.org/10.1158/1055-9965.EPI-22-0712.
Lam JUH, Rebolj M, Møller Ejegod D, Pedersen H, Rygaard C, Lynge E, et al. Human papillomavirus self-sampling for screening nonattenders: opt-in pilot implementation with electronic communication platforms. Int J Cancer. 2017;140:2212–9.
Ejegod DM, Pedersen H, Pedersen BT, Serizawa R, Bonde J. Operational experiences from the general implementation of HPV self-sampling to Danish screening non-attenders. Prev Med. 2022;160:107096.
Tranberg M, Bech BH, Blaakær J, Jensen JS, Svanholm H, Andersen B. HPV self-sampling in cervical cancer screening: the effect of different invitation strategies in various socioeconomic groups—a randomized controlled trial. Clin Epidemiol. 2018;10:1027–36.
Paolino M, Gago J, Le Pera A, Cinto O, Thouyaret L, Arrossi S. Adherence to triage among women with HPV-positive self-collection: a study in a middle-low income population in Argentina. ecancermedicalscience. 2020;14:1138.
Arbyn M, Peeters E, Benoy I, Vanden Broeck D, Bogers J, De Sutter P, et al. VALHUDES: A protocol for validation of human papillomavirus assays and collection devices for HPV testing on self-samples and urine samples. J Clin Virol. 2018;107:52–56.
Acknowledgements
Horizon 2020 Framework Programme for Research and Innovation of the European Commission and the US National Cancer Institute/NIH/DHHS.
Funding
MA was supported by the Horizon 2020 Framework Programme for Research and Innovation of the European Commission, through the RISCC Network (Grant No. 847845); Sciensano the employer of MA and SC received funding in the framework VALHUDES, a researcher-induced protocol for evaluation of HPV tests on self-samples (see Arbyn et al. [66]). MA and SC did not receive any financial or material benefit from this project. BV was supported by the Horizon 2020 Framework Programme for Research and Innovation of the European Commission, through the ELEVATE project (Grant No. 825747). PEC was supported by the intramural research program (ZIACP101237-01) of the US National Cancer Institute/NIH/DHHS.
Author information
Authors and Affiliations
Contributions
MA designed the study concept and protocol. MA, SC and BV contributed to the data extraction, and SC conducted the statistical analysis. SC and BV co-wrote the drafts of the manuscript. MA and PEC provided critical revisions for the manuscript, and all co-authors provided final approval to publish. All authors had full access to the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
PEC has received HPV tests and assays for research at a reduced or no cost from Roche, Becton Dickinson, Cepheid and Arbor Vita Corporation.
Ethics approval and consent to participate
This study used publicly available data extracted from published studies. Ethical approval by an Institutional Review Board was not required.
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Costa, S., Verberckmoes, B., Castle, P.E. et al. Offering HPV self-sampling kits: an updated meta-analysis of the effectiveness of strategies to increase participation in cervical cancer screening. Br J Cancer 128, 805–813 (2023). https://doi.org/10.1038/s41416-022-02094-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-02094-w
This article is cited by
-
Older women’s perceptions of HPV self-sampling and HPV-sampling performed by a midwife – a phenomenographic study
BMC Public Health (2024)
-
Evaluation of self-sampling-based cervical cancer screening strategy using HPV Selfy CE-IVD test coupled with home-collection kit: a clinical study in Italy
European Journal of Medical Research (2023)