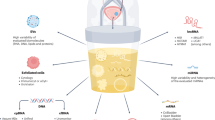
Abstract
Background
Non-invasive urine-based biomarkers can potentially improve current diagnostic and monitoring protocols for bladder cancer (BC). Here we assess the performance of earlier published biomarker panels for BC detection (BC-116) and monitoring of recurrence (BC-106) in combination with cytology, in two prospectively collected patient cohorts.
Methods
Of the 602 patients screened for BC, 551 were found eligible. For the primary setting, 73 patients diagnosed with primary BC (n = 27) and benign urological disorders, including patients with macroscopic haematuria, cystitis and/or nephrolithiasis (n = 46) were included. In total, 478 patients under surveillance were additionally considered (83 BC recurrences; 395 negative for recurrence). Urine samples were analysed with capillary electrophoresis-mass spectrometry. The biomarker score was estimated via support vector machine-based software.
Results
Validation of BC-116 biomarker panel resulted in 89% sensitivity and 67% specificity (AUCBC-116 = 0.82). A diagnostic score based on cytology and BC-116 resulted in good (AUCNom116 = 0.85) but not significantly better performance (P = 0.5672). A diagnostic score including BC-106 and cytology was evaluated (AUCNom106 = 0.82), significantly outperforming both cytology (AUCcyt = 0.72; P = 0.0022) and BC-106 (AUCBC-106 = 0.67; P = 0.0012).
Conclusions
BC-116 biomarker panel is a useful test for detecting primary BC. BC-106 classifier integrated with cytology showing >95% negative predictive value, might be useful for decreasing the number of cystoscopies during surveillance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data (except raw files) generated or analysed during this study are included in this article (and its supplementary information files). Raw data are available upon request from the corresponding author.
References
Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, Dominguez, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. 2022;81:75–94.
Sylvester RJ, Rodriguez O, Hernandez V, Turturica D, Bauerova L, Bruins HM, et al. European association of urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: an update from the EAU NMIBC guidelines panel. Eur Urol. 2021;79:480–8.
Tan WS, Rodney S, Lamb B, Feneley M, Kelly J. Management of non-muscle invasive bladder cancer: a comprehensive analysis of guidelines from the United States, Europe and Asia. Cancer Treat Rev. 2016;47:22–31.
Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet. 2016;388:2796–810.
Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur Urol. 2019;76:639–57.
Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49.
Maas M, Bedke J, Stenzl A, Todenhofer T. Can urinary biomarkers replace cystoscopy? World J Urol. 2019;37:1741–9.
Freifeld Y, Lotan Y. Effect of blue-light cystoscopy on contemporary performance of urine cytology. BJU Int. 2019;124:251–7.
Christensen E, Birkenkamp-Demtroder K, Nordentoft I, Hoyer S, van der Keur K, van Kessel KEM, et al. Liquid biopsy analysis of FGFR3 and PIK3CA hotspot mutations for disease surveillance in bladder cancer. Eur Urol. 2017;71:961–9.
Chakraborty A, Dasari S, Long W, Mohan C. Urine protein biomarkers for the detection, surveillance, and treatment response prediction of bladder cancer. Am J Cancer Res. 2019;9:1104–17.
Theodorescu D, Wittke S, Ross MM, Walden M, Conaway M, Just I, et al. Discovery and validation of new protein biomarkers for urothelial cancer: a prospective analysis. Lancet Oncol. 2006;7:230–40.
Schiffer E, Vlahou A, Petrolekas A, Stravodimos K, Tauber R, Geschwend JE, et al. Prediction of muscle-invasive bladder cancer using urinary proteomics. Clin Cancer Res. 2009;15:4935–43.
Frantzi M, Latosinska A, Fluhe L, Hupe MC, Critselis E, Kramer MW, et al. Developing proteomic biomarkers for bladder cancer: towards clinical application. Nat Rev Urol. 2015;12:317–30.
Frantzi M, Vlahou A. Ten years of proteomics in bladder cancer: progress and future directions. Bladder Cancer. 2017;3:1–18.
Frantzi M, van Kessel KEM, Zwarthoff EC, Marquez M, Rava M, Malats N, et al. Development and validation of urine-based peptide biomarker panels for detecting bladder cancer in a multi-center study. Clin Cancer Res. 2016;22:4077–86.
Krochmal M, van Kessel KEM, Zwarthoff EC, Belczacka I, Pejchinovski M, Vlahou A, et al. Urinary peptide panel for prognostic assessment of bladder cancer relapse. Sci Rep. 2019;9:7635.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pr Urol. 2005;2:416–22.
Mischak H, Allmaier G, Apweiler R, Attwood T, Baumann M, Benigni A, et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med. 2010;2:46ps42.
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. 2016;70:106–19.
Brierley JD, Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours, 8th ed. Chicester: John Wiley & Sons, Incorporated; 2016.
Argilés À, Siwy J, Duranton F, Gayrard N, Dakna M, Lundin U, et al. CKD273, a new proteomics classifier assessing CKD and its prognosis. PLoS ONE. 2013;8:e62837.
Neuhoff N, Kaiser T, Wittke S, Krebs R, Pitt A, Burchard A, et al. Mass spectrometry for the detection of differentially expressed proteins: a comparison of surface-enhanced laser desorption/ionization and capillary electrophoresis/mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:149–56.
Jantos-Siwy J, Schiffer E, Brand K, Schumann G, Rossing K, Delles C, et al. Quantitative urinary proteome analysis for biomarker evaluation in chronic kidney disease. J Proteome Res. 2009;8:268–81.
Coon JJ, Zurbig P, Dakna M, Dominiczak AF, Decramer S, Fliser D, et al. CE-MS analysis of the human urinary proteome for biomarker discovery and disease diagnostics. Proteom Clin Appl. 2008;2:964.
Siwy J, Mullen W, Golovko I, Franke J, Zurbig P. Human urinary peptide database for multiple disease biomarker discovery. Proteom Clin Appl. 2011;5:367–74.
DeLeo JM. Receiver operating characteristic laboratory (ROCLAB): software for developing decision strategies that account for uncertainty. In: 1993 (2nd) International Symposium on Uncertainty Modeling and Analysis. 1993; pp. 318–25. https://doi.org/10.1109/ISUMA.1993.366750.
Roobol MJ, van Vugt HA, Loeb S, Zhu X, Bul M, Bangma CH, et al. Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol. 2012;61:577–83.
Ng K, Stenzl A, Sharma A, Vasdev N. Urinary biomarkers in bladder cancer: a review of the current landscape and future directions. Urol Oncol. 2021;39:41–51.
Soria F, Droller MJ, Lotan Y, Gontero P, D’Andrea D, Gust KM, et al. An up-to-date catalog of available urinary biomarkers for the surveillance of non-muscle invasive bladder cancer. World J Urol. 2018;36:1981–95.
Frantzi M, Latosinska A, Belczacka I, Mischak H. Urinary proteomic biomarkers in oncology: ready for implementation? Expert Rev Proteom. 2019;16:49–63.
Konety B, Shore N, Kader AK, Porten S, Daneshmand S, Lough T, et al. Evaluation of cxbladder and adjudication of atypical cytology and equivocal cystoscopy. Eur Urol. 2019;76:238–43.
van der Heijden AG, Mengual L, Ingelmo-Torres M, Lozano JJ, van Rijt-van de Westerlo CCM, Baixauli M, et al. Urine cell-based DNA methylation classifier for monitoring bladder cancer. Clin Epigenet. 2018;10:71.
Grossman HB, Gomella L, Fradet Y, Morales A, Presti J, Ritenour C, et al. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol. 2007;178:62–7.
Laukhtina E, Shim SR, Mori K, D’Andrea D, Soria F, Rajwa P, et al. Diagnostic accuracy of novel urinary biomarker tests in non-muscle-invasive bladder cancer: a systematic review and network meta-analysis. Eur Urol Oncol. 2021;4:927–42.
Yabroff KR, Zhao J, de Moor JS, Sineshaw HM, Freedman AN, Zheng Z, et al. Factors associated with oncologist discussions of the costs of genomic testing and related treatments. J Natl Cancer Inst. 2020;112:498–506.
Wendt R, Thijs L, Kalbitz S, Mischak H, Siwy J, Raad J, et al. A urinary peptidomic profile predicts outcome in SARS-CoV-2-infected patients. EClinicalMedicine. 2021;36:100883.
Tofte N, Lindhardt M, Adamova K, Bakker SJL, Beige J, Beulens JWJ, et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8:301–12.
Funding
This work was supported by BioMedBC (752755; H2020-MSCA-IF-2016) a programme funded by the European Commission (EC) under Marie Sklodowska-Curie actions (MSCA) H2020 Work Programme. The specific role of the funding organisation (EC) was to financially support the acquisition of the data and part of the data analysis.
Author information
Authors and Affiliations
Contributions
AGVdH had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: LM, MF, AV, HM and AGVdH; acquisition of the data: LM, MF, MI-T and MV; analysis and interpretation of the data: LM, MF, MM, MI-T, MV; drafting of the manuscript: LM, MF, MM; critical revision of the manuscript for important intellectual content: LM, MF, MM, MI-T, MV, ASM, MCR, ZC, AA, AV, HM and AGVdH; statistical analysis: LM, MF and MM; obtaining funding: MF; administrative, technical or material support: LM, AA, AV, HM and AGVdH; supervision: LM, ASM, MCR, ZC, AA, HM and AGVdH; other/software validation: MF and MM.
Corresponding author
Ethics declarations
Competing interests
Prof. HM holds ownership interest in Mosaiques Diagnostics GmbH. Dr. MF and Dr. MM are employed by Mosaiques Diagnostics GmbH. The remaining authors declare no competing interests.
Ethics approval and consent to participate
Informed consent forms were obtained and adhered to Institutional Review Board-approved guidelines. Ethical approval for this study was obtained by the Ethics Committee in Medical School of Hannover (ID:3274–2016). The study was performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mengual, L., Frantzi, M., Mokou, M. et al. Multicentric validation of diagnostic tests based on BC-116 and BC-106 urine peptide biomarkers for bladder cancer in two prospective cohorts of patients. Br J Cancer 127, 2043–2051 (2022). https://doi.org/10.1038/s41416-022-01992-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01992-3