Summary
Background
The evidence on the efficacy of anticancer therapy is limited in older patients with metastatic colorectal cancer (mCRC). This retrospective analysis of phase III FIRE-3 trial assesses the efficacy of FOLFIRI plus either cetuximab or bevacizumab according to the patients’ age and sidedness of primary tumour.
Methods
The study endpoints overall response rate (ORR), progression-free survival (PFS) and overall survival (OS) were compared between younger (<65 years) and older (≥65 years) patients, followed by stratification according to primary tumour sidedness. ORR was compared using Fisher´s exact test, OS and PFS were estimated by the Kaplan–Meier method and compared using the log-rank test. Univariate Cox regression analyses assessed hazard ratios and 95% confidence intervals for OS and PFS.
Results
Overall, older patients with RAS WT tumours had a significantly shorter OS when compared to younger patients (25.9 months vs 29.3 months, HR 1.29; P = 0.02). Also the proportion of right-sided tumours was significantly greater in older patients (27.1% vs 17.9%; P = 0.029). Secondary resection rates were numerically higher in younger patients (25.4% vs. 17.6%, P = 0.068) than in older patients. This was primarily seen in the Cetuximab arm, where older patients underwent less likely resection (13.1% vs. 26%; P = 0.02). Older patients with left-sided tumours showed only a trend towards greater efficacy of cetuximab (HR 0.86; P = 0.38). In patients with right-sided primary tumours, older patients did not appear to benefit from cetuximab in contrast to younger patients (≥65 years: 16.6 months vs 23.6 months, HR 1.1; P = 0.87; <65 years: 21.9 months vs 16.4 months HR 1.5; P = 0.31).
Conclusions
In FIRE-3, OS was generally shorter in older patients in comparison to younger patients. This could be explained by the overrepresentation of right-sided tumours and a lower secondary resection rate in older patients. The efficacy of targeted therapy was dependent on tumour sidedness in older patients with RAS WT mCRC.
Clinical trial
FIRE-3 (NCT00433927).
Similar content being viewed by others
Introduction
Worldwide, colorectal cancer (CRC) is the third most frequent cancer and the second most frequent cause of cancer-related mortality [1]. In the last decades, CRC-associated mortality has been declining in patients from highly developed European countries. This decrease was, however, less pronounced in older people [2]. Several reports support the notion that mCRC in older patients is associated with a less favourable outcome than in younger patients [3, 4]. This finding is most likely multifactorial and can be explained by higher rates of comorbidities and frailty [5] as well as different treatment regimens throughout age cohorts [6]. In addition, the molecular biology underlying mCRC may be different in older patients with regard to tumour mutational load, epigenetic modifications or telomere dysfunction [7, 8].
Despite an increasing incidence of CRC at progressing age, a conclusive definition of older patients has not been established [9]. Patients at higher ages are hardly studied in clinical trials. Therefore, evidence-based therapy for this age group remains an unsolved issue. Current guidelines recommend stratification of patients to undergo intensified treatment not only by age but also by fitness [10, 11]. This stratification is essential, as older patients might not receive further treatment beyond first-line in contrast to younger patients.
Based on results from several randomised studies, treatment options for frail patients usually include fluoropyrimidines in combination with bevacizumab (FP/bev) [12,13,14,15,16]. In a predominantly older patient population, the sequential escalation from FP/bev to an irinotecan-based doublet plus bevacizumab at tumour progression failed to confirm non-inferiority to upfront combination therapy but showed different efficacy patterns according to molecular subgroups and gender [17]. Doublet chemotherapy showed a manageable safety profile but no clear survival benefit for elderly patients [18].
Data regarding the efficacy of anti-EGFR antibodies such as cetuximab [19] in older patients are, however, limited to retrospective or small prospective studies of often molecularly unselected patients.
We therefore aimed to investigate the impact of targeted biological therapies (cetuximab or bevacizumab) in combination with doublet chemotherapy (FOLFIRI) as a first-line regimen in a retrospective subgroup analysis of older patients with RAS WT metastatic CRC of the randomised phase III FIRE-3 trial (AIO KRK 0306). Here we examined not only the association of age and survival endpoints but also analysed treatment efficacy in relation to primary tumour location and patient age.
Methods
Study design and patients
Study design, eligibility criteria and treatment parameters of FIRE-3 have previously been reported [20]. Randomisation was done centrally via fax using permuted blocks of randomly varying sizes. Stratification was according to ECOG performance status (0–1 or 2), a number of metastatic sites (1 or >1), white blood cell count (<8 × 109 cells per L or ≥8 × 109 cells per L) and alkaline phosphatase concentration (<300 units per L or ≥300 units per L) [20]. The study was carried out in accordance with the Declaration of Helsinki. All patients provided written informed consent prior to their participation.
The primary endpoint was the investigator-assessed objective response rate (ORR, complete or partial response) according to the Response Evaluation Criteria in Solid Tumours (RECIST) criteria, version 1.0.5. Secondary endpoints included progression-free survival (PFS) and overall survival (OS). The statistical design of FIRE-3 has been described elsewhere [20].
In the post-hoc analysis of FIRE-3, efficacy results of a subgroup of patients with tumours that were wild-type at the RAS genes KRAS and NRAS exons 2–4 were presented (final RAS wild-type [21]).
In the final survival analysis 2021, the per-protocol population was defined and analysed [22]. In the present analysis, all patients with the final RAS wild-type of the post-hoc analysis [21] were included. Patients were grouped into cohorts over 65 plus 65 (≥65) or under 65 (<65) for age-related analysis.
Primary tumours originating from the caecum to the transverse colon were assigned to the right colon, and tumours of the splenic flexure to the rectum to the left colon, respectively.
Statistical analysis
Survival-based outcomes were analysed by the Kaplan–Meier method and described by median values. Comparisons of survival-based outcomes were conducted using log-rank tests and Cox regression analyses that were described as hazard ratios [17] with 95% confidence intervals (95% CI). Response rates and early tumour shrinkage were compared by Fisher’s exact test (two-sided). Differences in the depth of response between patients treated with FOLFIRI plus cetuximab and FOLFIRI plus bevacizumab were investigated with a two-sided Wilcoxon test. Where indicated, odds ratios (ORs) and hazard ratios (HRs) were calculated.
Toxicity was assessed using the NCI-CTCAE version 4.0 in all patients that received treatment within the study. Comparisons of symptomatic toxicities were conducted by Fisher’s exact test.
P-values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 25 for Windows (SPSS Inc, Chicago, IL).
Results
Study populations and baseline characteristics
In FIRE-3, eligible patients were aged 18–75 years [20,21,22]. Among 400 patients with final RAS wild-type mCRC, 199 were treated with FOLFIRI plus cetuximab and 201 with FOLFIRI plus bevacizumab. Subdivision of the whole study population at a cut-off of 65 years yielded nearly equally sized patient cohorts. The median age of the investigated population was 64 years. The younger age cohort (<65 years) contained 201 patients (50.2%), while 199 patients (49.8%) were included in the older cohort (≥65 years).
In the overall study population, 77% of patients presented with left-sided and 22.5% with right-sided primary tumours, while in 0.5% of patients primary tumour location could not be determined. Primary tumours were left-sided in 72.4% of older (age ≥65 years) and in 81.6% of younger patients (age <65 years). As a result, the proportion of right-sided tumours was significantly greater in older patients (27.1% vs 17.9%; P = 0.029). Other patient- and tumour-related characteristics were balanced between age groups. For baseline characteristics according to age subgroups please refer to Table 1.
The overall response rate was assessable in 353 of 400 patients, while 47 patients were not evaluable as previously reported [20]. The safety profiles in both treatment groups were consistent with the known side-effects of the individual study drugs (Supplementary Appendix. A). Haematological side-effects were numerically higher in younger compared to older patients with regard to Grade-3 (16.9% vs 22.6%) and Grade-4 (4.5% vs 6.0%) toxicities (Supplementary Appendix. A).
Prognostic impact of age in the RAS wild-type population
In the primary analysis of the final RAS wild-type population, ORR and PFS were comparable between the treatment groups, but OS was longer in patients treated with FOLFIRI plus cetuximab [20].
In the overall study population (n = 400), patients with final RAS wild-type tumours aged ≥65 years had a significantly shorter median OS, when compared to younger patients (25.9 months vs 29.3 months, P = 0.02, Table 2). In particular, older patients had a markedly shorter survival in the cetuximab arm (27.1 months vs 33.1 months, HR 1.51; 95% CI, 1.10–2.06; P = 0.009). In contrast, no age-related effect was observed in the bevacizumab arm (26.0 months vs 25.6 months, HR 1.1; 95% CI, 0.82–1.47; P = 0.53, Table 2).
Impact of age on treatment efficacy of cetuximab and bevacizumab in left- and right-sided primary tumours
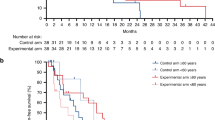
Considering all tumour localisations together, the comparison of OS by treatment arm clearly demonstrated that FOLFIRI plus cetuximab was markedly superior to FOLFIRI plus bevacizumab in patients aged ≤65 years (OS 33.1 months vs 25.6 months; HR 0.67; 95% CI, 0.5–0.91; P = 0.012, Fig. 1a). Within the older patients, no significant difference in efficacy between cetuximab- and bevacizumab-treated patients could be observed (27.1 vs. 26.0 months; HR 0.89; 95% CI, 0.66–1.2; P = 0.46, Table 2). The analysis of ORR in younger patients showed a markedly greater benefit from cetuximab compared to bevacizumab (76.2% vs 62.9%, P = 0.076). In older patients ORR was only numerically greater in the cetuximab arm (77.9% vs 66.3%, P = 0.13) and this effect did not reach the level of statistical significance (Table 2). For further clarification, the age subgroup analysis was extended to sidedness (left versus right) of colorectal cancer. In the overall study population, patients with left-sided primary tumours lived longer compared to patients with right-sided primary tumours (30.8 months versus 21.2 months, P < 0.01). Only 90 patients with right-sided primary tumours were evaluated in the present analysis (36 patients aged <65 years and 54 patients aged ≥65 years, Table 2).
In the left-sided, RAS wild-type, colorectal cancer population <65 years, OS was significantly longer in cetuximab- compared to bevacizumab-treated patients (38.2 months vs. 28.2 months; HR 0.62, 95% CI 0.44–0.88; P = 0.006, Fig. 1). PFS and ORR were only numerically greater in the cetuximab arm (PFS: 12 vs 10.3 months, P = 0.3; 78.1% vs 65.8%, P = 0.11, Table 2).
In patients with right-sided, RAS wild-type tumours <65 years, the difference between treatment groups lost the level of significance and cetuximab led only numerically to superiority (21.9 vs 16.4, P = 0.31). PFS and ORR were comparable between treatment groups (PFS 7.2 vs 7.2, P = 0.19; 63.6% vs 52.4%, P = 0.71, Table 2).
For patients older than 65 years with left-sided CRC, the increase of median OS in cetuximab- compared to bevacizumab-treated patients was just numerically evident (33.2 vs 27.5 months, HR 0.86; 95% CI, 0.6–1.2; P = 0.38, Table 2) and also ORR did not reach level of significance (80.6% vs 70.0%, P = 0.22, Table 2). PFS did not significantly differ between the cetuximab group and the bevacizumab group (Table 2). Importantly, older patients with right-sided primaries did not appear to benefit from cetuximab as compared to bevacizumab with regard to median OS (16.6 months vs 23.6 months, HR 1.1; 95% CI, 0.6–1.87; P = 0.87, Fig. 1b, Table 2). PFS and ORR resembled between treatment groups (PFS 8.0 vs 9.3, P = 0.44; 68.4% vs 57.7%, P = 0.54, Table 2). The median OS outcomes in older patients contrast those in younger patients (OS 21.9 months versus 16.4 months, HR 0.68; 95% CI, 0.32–1.44; P = 0.31, Table 2).
Potential drivers accounting for differences in efficacy
To rule out the possibility that less therapy may account for the shorter survival of older patients, the number of treatment cycles within the study as well as further lines of therapy were examined.
Older patients received comparable numbers of treatment cycles with FOLFIRI/cetuximab vs FOLFIRI/bevacizumab (12.7 vs 12.9) compared to younger patients (13.1 vs 14.5). Also the percentage of patients receiving second- and third-line chemotherapy was not significantly different between younger and older patients (Table 3).
To account for potential drivers of the inferior outcomes of older patients, further exploratory analyses were conducted. Exploration of early tumour shrinkage (ETS) and depth of response (DpR) according to age groups showed no statistical difference (Table 3). In addition, patients were classified according to clinically significant cetuximab-induced skin toxicity, but no significant difference in severity was observed in older patients who received cetuximab (Table 3).
Secondary resection rates were numerically higher in younger patients (25.4% vs. 17.6%, P = 0.068) than in older patients. Mainly in the FOLFIRI + cetuximab arm, older patients underwent less likely resection (13.1% vs. 26%; P = 0.02, Table 3). Both, older and younger patients with resectable disease that actually underwent resection had superior overall survival (44.3 months vs 23.8 months, HR 0.44; 95% CI, 0.29–0.67; P < 0.005 and for younger patients 43.7 months vs 24.5 months, HR 0.46; 95% CI, 0.31–0.67; P < 0.005, Fig. 2), notably patients with left-sided tumours (data not shown). In patients who underwent resection overall survival between younger and older patients was similar (43.7 months vs 44.3 months, HR 1.3; 95% CI, 0.8–1.22; P = 0.29).
Discussion
The present analysis of the FIRE-3 trial was performed as a post-hoc analysis of patients with RAS wild-type mCRC grouped in two age cohorts. Two questions were investigated: Firstly, to what extent overall survival depends on age; secondly, which targeted therapy, as an addition to first-line treatment with FOLFIRI, should be preferred in relation to age and sidedness of tumour. In this context, it is relevant to point out that patients included in FIRE-3 were deemed fit for combination chemotherapy plus targeted therapy independent of age. Hence, the comparison of an older versus a younger age group in this study does not necessarily reflect the comparison of a frail versus a fit subgroup of patients.
Comparing different age cohorts in the overall study population of FIRE-3, OS was significantly shorter in the age cohort ≥65 years as compared to younger patients. This effect is likely multifactorial. Firstly, it could be attributed to a higher proportion of right-sided tumours in older patients (Table 1). As expected, older patients (≥65 years) in FIRE-3 showed a higher proportion of right-sided tumours than younger patients. In accordance with the published literature, right-sided primary tumour location was associated with markedly shorter OS compared to left-sided primary tumour location when considering the overall study population. This effect was observed in the younger as well as in the older patient cohort. Secondly, younger patients showed a trend toward a higher secondary resection rate. Secondary resection is associated with a better OS [23]. To assess, if older patients profit as same as younger patients from secondary resection, OS was compared between younger and older resected patients and showed no difference. So, in the FIRE-3 population, secondary resection in older patients was safely performed and resulted in better OS.
Evaluation of the study population according to targeted therapy demonstrated that significantly shorter survival in older versus younger patients was observed only in the cetuximab arm (HR 1.5, P < 0.01), but not in bevacizumab-treated patients (HR 1.1, P = 0.53) of FIRE-3. This finding may point to an age-related effect of anti-EGFR directed therapy. It has not been reported previously and thus needs confirmation. A similar observation was made by Garcia-Alfonso et al. 2021 but in the meta-analysis, factors like worse ECOG and a lower percentage of active treatment after first-line therapy have contributed to the shorter OS of older patients [24]. In the present analysis, there were no differences in ECOG or lower percentage of second or third-line therapy but older patients treated with cetuximab had a lower secondary resection rate compared to older patients treated with bevacizumab (Table 3).
Previous reports have demonstrated that combination chemotherapy may improve PFS and OS in younger as well as in older patients [18, 25, 26]. In the present study, combination chemotherapy was used as a backbone treatment with the expectation that tolerability and efficacy would be acceptable and comparable through different age groups. The present evaluation asks the question if this assumption also holds for targeted therapy with specific regard to anti-EGFR- versus anti-VEGF-directed therapy.
Previous studies support the notion that the addition of bevacizumab to FP significantly improved PFS in older patients, while the effect on OS remains less clear [12,13,14,15,16]. There is less evidence on cetuximab-based combination therapy in first-line treatment of older mCRC patients. A combination of cetuximab with single-agent chemotherapy was shown to be more effective in KRAS WT mCRC patients [27]. In addition, non-interventional and retrospective investigations revealed the efficacy of cetuximab plus irinotecan in older patients with pre-treated KRAS WT mCRC and had a similar safety profile compared to younger patients [28, 29]. However, in the PRIME study, the addition of Panitumumab to first-line treatment with FOLFOX-4 showed OS benefit in the cohort of RAS wild-type patients <65 years but not for older patients [30].
In the present investigation of older patients (≥65 years) with RAS WT mCRC, there were no significant differences between cetuximab- or bevacizumab-based therapy with regard to ORR, PFS or OS. This result is in clear contrast to younger patients (<65 years) as well as to the unselected population where FOLFIRI plus cetuximab led to a significantly longer OS when compared to FOLFIRI plus bevacizumab [20]. Translating these results into clinical practice would mean that in older patients with RAS WT mCRC there is no favoured targeted therapy, since both anti-EGFR- and anti-VEGF-directed treatment yields comparable results. Due to a major survival advantage (HR 0.67, P = 0.012), there is, however, a strong recommendation to use first-line cetuximab in younger patients.
Primary tumour sidedness is not only an important prognostic factor in metastatic colorectal cancer, but it is also predictive as it affects the response to anti-EGFR-directed therapies. Thus it was shown that cetuximab-based therapy resulted in better outcomes in patients with left- compared to right-sided tumours KRAS wild-type mCRC [2, 31, 32].
Good evidence exists that patients with right-sided mCRC do either not benefit or even derive a disadvantage from anti-EGFR-directed treatment [33, 34].
A strong and statistically significant superiority of cetuximab over bevacizumab was specifically shown in patients with left-sided mCRC aged <65 years. Older patients with left-sided primary tumours treated with cetuximab had a not significantly prolonged OS when compared to bevacizumab. In contrast, in patients with right-sided primaries no benefit from cetuximab was observed independent of age.
Potential limitations of our study include its retrospective nature as well as the limited patient numbers in analysed subgroups. This issue becomes relevant specifically with regard to the subgroup of right-sided cancers, which is notably smaller than left-sided ones [3, 8, 35].
For the purpose of comparing equally sized groups, the present study elected to choose an age cut-off of 65 years. This had several reasons. Firstly, eligible patients in FIRE-3 were aged 18–75 years so subdivision of the study population at a cut-off of 65 years resulted in nearly equally sized patient cohorts. While patients aged ≥65 years clearly do not represent an elderly population per se, there is no widely accepted cut-off that defines the so-called elderly population in patients with metastatic colorectal cancer. Nevertheless, the present observations must be considered mainly as hypothesis-generating and clearly require confirmation by prospective studies.
Treatment recommendations for older patients with metastatic disease should focus not only on the molecular biology of the tumour but must also allow for the development of personalised multidisciplinary strategies, considering fitness, comorbidities and expectations of the patient as well as anticipated side-effects of antineoplastic therapy.
Conclusions
The results presented here suggest that older patients have a shorter OS despite intensive treatment. This observation could be explained by the overrepresentation of right-sided tumours in elderly patients. Furthermore, in our cohort, older patients were less likely to undergo secondary resection. However, secondary resection was equally beneficial in older and younger patients.
According to our analyses, for older patients, no favoured targeted therapy emerged as both anti-EGFR- and anti-VEGF-directed treatment yielded comparable results. These findings could be taken into account in the multidisciplinary management of older patients suffering from RAS wild-type colorectal cancer.
Data availability
All authors had access to the data published in this paper. The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–29.
Kotake K, Asano M, Ozawa H, Kobayashi H, Sugihara K. Tumour characteristics, treatment patterns and survival of patients aged 80 years or older with colorectal cancer. Colorectal Dis. 2015;17:205–15.
Patel SS, Nelson R, Sanchez J, Lee W, Uyeno L, Garcia-Aguilar J, et al. Elderly patients with colon cancer have unique tumor characteristics and poor survival. Cancer. 2013;119:739–47.
Boakye D, Rillmann B, Walter V, Jansen L, Hoffmeister M, Brenner H. Impact of comorbidity and frailty on prognosis in colorectal cancer patients: a systematic review and meta-analysis. Cancer Treat Rev. 2018;64:30–9.
Turner NJ, Haward RA, Mulley GP, Selby PJ. Cancer in old age–is it inadequately investigated and treated? BMJ. 1999;319:309–12.
DePinho RA. The age of cancer. Nature. 2000;408:248–54.
Arai T, Takubo K. Clinicopathological and molecular characteristics of gastric and colorectal carcinomas in the elderly. Pathol Int. 2007;57:303–14.
Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol. 2015;26:288–300.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
Network. NCC. Colon Cancer (Version 2.2021). 2021. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–85.
Aparicio T, Bouché O, Taieb J, Maillard E, Kirscher S, Etienne PL, et al. for PRODIGE 20 Investigators. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: a randomized phase II trial-PRODIGE 20 study results. Ann Oncol. 2018;29:133–8.
Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–705.
Price TJ, Zannino D, Wilson K, Simes RJ, Cassidy J, Van Hazel GA, et al. Bevacizumab is equally effective and no more toxic in elderly patients with advanced colorectal cancer: a subgroup analysis from the AGITG MAX trial: an international randomised controlled trial of Capecitabine, Bevacizumab and Mitomycin C. Ann Oncol. 2012;23:1531–6.
Pinto C, Antonuzzo L, Porcu L, Aprile G, Maiello E, Masi G, et al. Efficacy and safety of bevacizumab combined with fluoropyrimidine monotherapy for unfit or older patients with metastatic colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer. 2017;16:e61–e72.
Modest DP, Fischer von Weikersthal L, Decker T, Vehling-Kaiser U, Uhlig J, Schenk M, et al. Sequential versus combination therapy of metastatic colorectal cancer using fluoropyrimidines, irinotecan, and bevacizumab: a randomized, controlled study-XELAVIRI (AIO KRK0110). J Clin Oncol. 2019;37:22–32.
Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–59.
Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317:2392–401.
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75.
Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426–34.
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Kaiser F, Al-Batran S-E. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer. 2021;124:587–94.
Modest DP, Denecke T, Pratschke J, Ricard I, Lang H, Bemelmans M, et al. Surgical treatment options following chemotherapy plus cetuximab or bevacizumab in metastatic colorectal cancer—central evaluation of FIRE-3. Eur J Cancer. 2018;88:77–86.
García-Alfonso P, Díaz-Rubio E, Abad A, Carrato A, Massutí B, Ortiz-Morales MJ, et al. First-line biological agents plus chemotherapy in older patients with metastatic colorectal cancer: a retrospective pooled analysis. Drugs Aging. 2021;38:219–31.
Folprecht G, Seymour MT, Saltz L, Douillard JY, Hecker H, Stephens RJ, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26:1443–51.
Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–91.
Sastre J, Grávalos C, Rivera F, Massuti B, Valladares-Ayerbes M, Marcuello E, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist. 2012;17:339–45.
Bouchahda M, Macarulla T, Spano JP, Bachet JB, Lledo G, Andre T, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol. 2008;67:255–62.
Fornaro L, Baldi GG, Masi G, Allegrini G, Loupakis F, Vasile E, et al. Cetuximab plus irinotecan after irinotecan failure in elderly metastatic colorectal cancer patients: clinical outcome according to KRAS and BRAF mutational status. Crit Rev Oncol Hematol. 2011;78:243–51.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–55.
von Einem JC, Heinemann V, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140:1607–14.
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98.
Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3:194–201.
Venook AP, Niedzwiecki D, Innocenti F, Fruth B, Greene C, O’Neil BH, et al. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405(Alliance). J Clin Oncol. 2016;34:abstr 3504.
Wang F, Bai L, Liu TS, Yu YY, He MM, Liu KY, et al. Right-sided colon cancer and left-sided colorectal cancers respond differently to cetuximab. Chin J Cancer. 2015;34:384–93.
Acknowledgements
Parts of this manuscript were presented as poster at ASCO Annual Meeting 2019.
Funding
The legal funder (sponsor) of the trial is University Hospital, LMU Munich. This work was supported by grants from Pfizer GmbH, Germany, and Merck KGaA, Darmstadt, Germany. The funding sources had a role in the design and conduct of the study, and in the collection, management, analysis and interpretation of the data. Prior to 2009, cetuximab was supplied by Merck Serono GmbH, an affiliate of Merck KGaA, Darmstadt, Germany. Merck KGaA reviewed the paper for medical accuracy only and had no role in the decision to submit the paper for publication. The authors are fully responsible for the content of this paper, and the views and opinions described in the publication solely reflect those of the authors. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
LF, SS and VH: data analysis and interpretation, statistical analysis and paper writing. WS, DPM, SS and VH: writing of the protocol, data collection and interpretation. AJ and TK: analysis of tumour specimen, data interpretation and paper writing. SH: protocol writing and statistical analysis. LFvW, TD, AK, FK, S-EA-B, TH, CL, CK, GS, FK, MS, CG-J, JU, BP, CD, AS, LW and KH.: data collection, interpretation and paper writing.
Corresponding author
Ethics declarations
Competing interests
LEF: No conflicts of interests. SS: Honoraria: AMGEN, Bayer, BMS, ESAI, Lilly, Merck KGaA, MSD, Pierre-Fabre, Roche, Sanofi, Servier, Taiho, Takeda, Financial relationships: Merck KGaA, Pierre-Fabre, Servier, Roche. Advisory role: AMGEN, Bayer, BMS, ESAI, Lilly, Merck KGaA, MSD, Pierre-Fabre, Roche, Sanofi, Servier, Taiho, Takeda. LFvW: Honoraria: Novartis, Lilly, Piere-Fabre and Roche Pharma AG. DPM: Honoraria: Merck Serono, Amgen, Roche, Servier, BMS, MSD, Pierre Fabre, Onkowissen.de, Taiho, Sanofi, Eli Lilly. Consulting or Advisory Role: Merck Serono, Amgen, Bayer, Incyte, Servier, BMS, Onkowissen.de. Research Funding: Amgen (Inst), Servier (Inst). Travel, Accommodations, Expenses: Amgen, Merck Serono, Servier, Bristol-Myers Squibb. TD: Advisory Role: Roche and Novartis. AK: Honoraria: Merck and Roche. FK: Advisory Role: Elsevier, Astellas, MSD, Novartis, Servier, GSK. S-EA-B: No conflicts of interests. TH; No conflicts of interests. CL: No conflicts of interests. CK: No conflicts of interests. GS: No conflicts of interests. FK: No conflicts of interests. MS: No conflicts of interests. WS: No conflicts of interests. CG-J: Honoraria/Travel: Roche. JU: Adboards/Workshops: Roche, Amgen, Servier, MSD, Bristol-Myers Squibb, Sanofi, Merck, Celgene, Novartis, Janssen-Cilag, Boehringer-Ingelheim und Bayer, Biogene. BP: No conflicts of interests. CD: Financial relationships: Janssen, Novartis, Celgene, Incyte, Abbvie, Bayer, Merck. AS: Honoraria: Roche and Servier. Travel: Roche, Merck KGaA, MSD Sharp & Dohme, Pfizer and Amgen. LW: Honoraria: Roche and Servier. KH: No conflicts of interests. SH; No conflicts of interests. AJ: No conflicts of interests. TK: Consulting/Advisory: Amgen, AstraZeneca, BMS, Merck KGaA, MSD, Novartis, Pfizer, Roche. Research Funding: Merck and Roche. Advisory Role: Merck KGaA, Astra Zeneca. VH: Honoraria: Merck, Amgen, Roche, Sanofi, SIRTEX, Servier, Pfizer, Pierre-Fabre, Astra-Zeneca. Consulting: Merck, Amgen, Roche, Sanofi, SIRTEX, BMS; MSD, Novartis, Boehringer Ingelheim, Servier, Pierre-Fabre, Celgene, Terumo. Research funding (for the institution): Merck, Amgen, Roche, Sanofi, Pfizer, Boehringer-Ingelheim, Sirtex, Bayer, Servier. Travel accommodation expenses: Merck, Roche, Amgen, SIRTEX, Bayer, Servier.
Ethics approval and consent to participate
The protocol and informed consent forms were approved by the ethic committee of the Medical Faculty of the Ludwig-Maximilians-University (reference number: 370-06). Informed consent was obtained from all subjects prior to participating in the study. The study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fischer, L.E., Stintzing, S., von Weikersthal, L.F. et al. Efficacy of FOLFIRI plus cetuximab vs FOLFIRI plus bevacizumab in 1st-line treatment of older patients with RAS wild-type metastatic colorectal cancer: an analysis of the randomised trial FIRE-3. Br J Cancer 127, 836–843 (2022). https://doi.org/10.1038/s41416-022-01854-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01854-y