Abstract
Background
Circulating tumour DNA (ctDNA) has been spotlighted as an attractive biomarker because of its easy accessibility and real-time representation of tumour genetic profile. However, the clinical utility of longitudinal ctDNA monitoring has not been clearly defined.
Methods
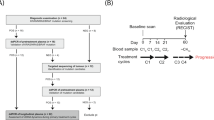
Serial blood samples were obtained from metastatic colorectal cancer patients undergoing first-line chemotherapy. ctDNA was sequenced using a targeted next-generation sequencing platform which included 106 genes. Changes in ctDNA profile and treatment outcome were comprehensively analysed.
Results
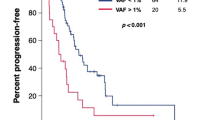
A total of 272 samples from 62 patients were analysed. In all, 90.3% of patients had detectable ctDNA mutation before treatment. ctDNA clearance after chemotherapy was associated with longer progression-free survival which was independent of radiological response (adjusted hazard ratio 0.22, 95% confidence interval 0.10–0.46). Longitudinal monitoring was able to detect ctDNA progression which preceded radiological progressive disease (PD) in 58.1% (median 3.3 months). Diverse resistant mutations (34.9%) and gene amplification (7.0%) at the time of PD were discovered. For 16.3% of the PD patients, the newly identified mutations could be potential candidates of targeted therapy or clinical trial.
Conclusion
ctDNA profile provided a more accurate landscape of tumour and dynamic changes compared to radiological evaluation. Longitudinal ctDNA monitoring may improve personalised treatment decision-making.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this article and the supplementary information files.
References
Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20:71–88.
Alix-Panabieres C, Pantel K. Liquid biopsy: from discovery to clinical application. Cancer Discov. 2021;11:858–73.
Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N. Engl J Med. 2018;379:1754–65.
Cescon DW, Bratman SV, Chan SM, Siu LL. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. 2020;1:276–90.
Cheng ML, Pectasides E, Hanna GJ, Parsons HA, Choudhury AD, Oxnard GR. Circulating tumor DNA in advanced solid tumors: Clinical relevance and future directions. CA Cancer J Clin. 2021;71:176–90.
Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol. 2018;36:1631–41.
Scholer LV, Reinert T, Orntoft MW, Kassentoft CG, Arnadottir SS, Vang S, et al. Clinical implications of monitoring circulating tumor DNA in patients with colorectal cancer. Clin Cancer Res. 2017;23:5437–45.
Tie J, Cohen JD, Wang Y, Li L, Christie M, Simons K, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut. 2019;68:663–71.
Coombes RC, Page K, Salari R, Hastings RK, Armstrong A, Ahmed S, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25:4255–63.
Strickler JH, Loree JM, Ahronian LG, Parikh AR, Niedzwiecki D, Pereira AAL, et al. Genomic landscape of cell-free DNA in patients with colorectal cancer. Cancer Discov. 2018;8:164–73.
Cao H, Liu X, Chen Y, Yang P, Huang T, Song L, et al. Circulating tumor DNA is capable of monitoring the therapeutic response and resistance in advanced colorectal cancer patients undergoing combined target and chemotherapy. Front Oncol. 2020;10:466.
Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:827.
Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med. 2014;20:430–5.
Vidal J, Muinelo L, Dalmases A, Jones F, Edelstein D, Iglesias M, et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann Oncol. 2017;28:1325–32.
Grasselli J, Elez E, Caratu G, Matito J, Santos C, Macarulla T, et al. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol. 2017;28:1294–301.
Thierry AR, Pastor B, Jiang ZQ, Katsiampoura AD, Parseghian C, Loree JM, et al. Circulating DNA demonstrates convergent evolution and common resistance mechanisms during treatment of colorectal cancer. Clin Cancer Res. 2017;23:4578–91.
Hsu HC, Lapke N, Wang CW, Lin PY, You JF, Yeh CY, et al. Targeted sequencing of circulating tumor DNA to monitor genetic variants and therapeutic response in metastatic colorectal cancer. Mol Cancer Ther. 2018;17:2238–47.
Leighl NB, Page RD, Raymond VM, Daniel DB, Divers SG, Reckamp KL, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res. 2019;25:4691–4700.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Meddeb R, Pisareva E, Thierry AR. Guidelines for the preanalytical conditions for analyzing circulating cell-free DNA. Clin Chem. 2019;65:623–33.
Salvianti F, Gelmini S, Costanza F, Mancini I, Sonnati G, Simi L, et al. The pre-analytical phase of the liquid biopsy. N. Biotechnol. 2020;55:19–29.
Volckmar AL, Sultmann H, Riediger A, Fioretos T, Schirmacher P, Endris V, et al. A field guide for cancer diagnostics using cell-free DNA: from principles to practice and clinical applications. Genes Chromosomes Cancer. 2018;57:123–39.
Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv arXiv. 2013;1303:3997v2.
Pereira B, Chen CT, Goyal L, Walmsley C, Pinto CJ, Baiev I, et al. Cell-free DNA captures tumor heterogeneity and driver alterations in rapid autopsies with pre-treated metastatic cancer. Nat Commun. 2021;12:3199.
Parikh AR, Mojtahed A, Schneider JL, Kanter K, Van Seventer EE, Fetter IJ, et al. Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clin Cancer Res. 2020;26:1877–85.
Oikkonen J, Zhang K, Salminen L, Schulman I, Lavikka K, Andersson N, et al. Prospective longitudinal ctDNA workflow reveals clinically actionable alterations in ovarian cancer. JCO Precis Oncol. 2019;3:1–12.
Jacob S, Davis AA, Gerratana L, Velimirovic M, Shah AN, Wehbe F, et al. The use of serial circulating tumor DNA to detect resistance alterations in progressive metastatic breast cancer. Clin Cancer Res. 2021;27:1361–70.
Lim Y, Kim S, Kang JK, Kim HP, Jang H, Han H, et al. Circulating tumor DNA sequencing in colorectal cancer patients treated with first-line chemotherapy with anti-EGFR. Sci Rep. 2021;11:16333.
Baldus SE, Schaefer KL, Engers R, Hartleb D, Stoecklein NH, Gabbert HE. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res. 2010;16:790–9.
Mao C, Wu XY, Yang ZY, Threapleton DE, Yuan JQ, Yu YY, et al. Concordant analysis of KRAS, BRAF, PIK3CA mutations, and PTEN expression between primary colorectal cancer and matched metastases. Sci Rep. 2015;5:8065.
Scherer F. Capturing tumor heterogeneity and clonal evolution by circulating tumor DNA profiling. In: Schaffner F, Merlin F, & von Bubnoff N, editors. Tumor liquid biopsies. Cham: Springer International Publishing; 2020. 213-30.
Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov. 2016;6:147–53.
Tie J, Kinde I, Wang Y, Wong HL, Roebert J, Christie M, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol. 2015;26:1715–22.
Jones RP, Pugh SA, Graham J, Primrose JN, Barriuso J. Circulating tumour DNA as a biomarker in resectable and irresectable stage IV colorectal cancer; a systematic review and meta-analysis. Eur J Cancer. 2021;144:368–81.
Kang JK, Heo S, Kim HP, Song SH, Yun H, Han SW, et al. Liquid biopsy-based tumor profiling for metastatic colorectal cancer patients with ultra-deep targeted sequencing. PLoS ONE. 2020;15:e0232754.
Kato S, Schwaederle MC, Fanta PT, Okamura R, Leichman L, Lippman SM, et al. Genomic assessment of blood-derived circulating tumor DNA in patients with colorectal cancers: correlation with tissue sequencing, therapeutic response, and survival. JCO Precis Oncol. 2019;3:1–16.
Bando H, Nakamura Y, Taniguchi H, Shiozawa M, Yasui H, Esaki T, et al. Impact of a metastatic site on circulating tumor DNA (ctDNA) analysis in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol. 2021;39:3554.
Acknowledgements
We thank all the patients and their caregivers for participating in this study.
Funding
This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C2282).
Author information
Authors and Affiliations
Contributions
S-WH and T-YK conceived the study and have oversight as principal investigators and designed the protocol. SK conducted the data management, clinical analysis and wrote the first draft of the manuscript. YL interpreted the work and wrote the first draft of the manuscript. J-KK, H-PK, HR and SK conducted the bioinformatic analysis. S-YJ and KJP contributed to data acquisition. All authors critically reviewed the drafts and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
YL, J-KK, H-PK, HR and SYK are employees of IMBdx. DB has owned stock of IMBdx and Celemics. DB and S-WH have received research fund from IMBdx. T-YK is the founder of IMBdx. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted under the approval of the Institutional Review Board (IRB) of SNUH (IRB number: 1805-049-944) and in accordance with the Declaration of Helsinki in biomedical research involving human subjects. All participants provided written informed consent prior to any study procedures.
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, S., Lim, Y., Kang, JK. et al. Dynamic changes in longitudinal circulating tumour DNA profile during metastatic colorectal cancer treatment. Br J Cancer 127, 898–907 (2022). https://doi.org/10.1038/s41416-022-01837-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01837-z