Abstract
Background
Blood-based biomarkers used for colorectal cancer screening need to be developed and validated in appropriate screening populations. We aimed to develop a cancer-associated protein biomarker test for the detection of colorectal cancer in a screening population.
Methods
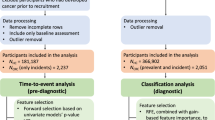
Participants from the Danish Colorectal Cancer Screening Program were recruited. Blood samples were collected prior to colonoscopy. The cohort was divided into training and validation sets. We present the results of model development using the training set. Age, sex, and the serological proteins CEA, hsCRP, TIMP-1, Pepsinogen-2, HE4, CyFra21-1, Galectin-3, ferritin and B2M were used to develop a signature test to discriminate between participants with colorectal cancer versus all other findings at colonoscopy.
Results
The training set included 4048 FIT-positive participants of whom 242 had a colorectal cancer. The final model for discriminating colorectal cancer versus all other findings at colonoscopy had an AUC of 0.70 (95% CI: 0.66–0.74) and included age, sex, CEA, hsCRP, HE4 and ferritin.
Conclusion
The performance of the biomarker signature in this FIT-positive screening population did not reflect the positive performance of biomarker signatures seen in symptomatic populations. Additional biomarkers are needed if the serological biomarkers are to be used as a frontline screening test.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91.
Gawel SH, Lucht M, Gomer H, Treado P, Christensen IJ, Nielsen HJ, et al. Evaluation of algorithm development approaches: Development of biomarker panels for early detection of colorectal lesions. Clin Chim Acta. 2019;498:108–15.
Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17–22.
Young JP, Win AK, Rosty C, Flight I, Roder D, Young GP, et al. Rising incidence of early-onset colorectal cancer in Australia over two decades: report and review. J Gastroenterol Hepatol. 2015;30:6–13.
Vuik FER, Nieuwenburg SAV, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820–6.
Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med. 2017;65:311–5.
Kubisch CH, Crispin A, Mansmann U, Göke B, Kolligs FT. Screening for colorectal cancer is associated with lower disease stage: a population-based study. Clin Gastroenterol Hepatol. 2016;14:1612–8.
Friedrich K, Grüter L, Gotthardt D, Eisenbach C, Stremmel W, Scholl SG, et al. Survival in patients with colorectal cancer diagnosed by screening colonoscopy. Gastrointest Endosc. 2015;82:133–7.
Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl J Med. 2012;366:687–96.
Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155:1383–91. e5
Sullivan T, Sullivan R, Ginsburg OM. Screening for cancer: considerations for low- and middle-income countries. In: Gelbrand, Jha P, Sankaranarayanan R, et al. editors. Cancer: disease control priorities. Third Edition, Vol. 3, Chapter 12, 2015. Page 211–222.
Morris A, You YN, Sullivan PS. Young-onset colorectal cancer: American Cancer Society issues new screening guidelines; 2020. https://bulletin.facs.org/2020/02.
Toes-Zoutendijk E, van Leerdam ME, Dekker E, van Hees F, Penning C, Nagtegaal I, et al. Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology. 2017;152:767–75.
Mertz-Petersen M, Piper TB, Kleif J, Ferm L, Christensen IJ, Nielsen HJ, et al. Triage for selection to colonoscopy? Eur J Surg Oncol. 2018;44:1539–41.
Klabunde C, Blom J, Bulliard JL, Garcia M, Hagoel L, Mai V, et al. Participation rates for organized colorectal cancer screening programmes: an international comparison. J Med Screen. 2015;22:119–26.
Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: an update. World J Gastroenterol. 2017;23:3632–42.
Njor SH, Friis-Hansen L, Andersen B, Søndergaard B, Linnemann D, Jørgensen JCR, et al. Three years of colorectal cancer screening in Denmark. Cancer Epidemiol. 2018;57:39–44.
Osborne JM, Flight I, Wilson CJ, Chen G, Ratcliffe J, Young GP. The impact of sample type and procedural attributes on relative acceptability of different colorectal cancer screening regimens. Patient Prefer Adherence. 2018;12:1825–36.
Liles EG, Coronado GD, Perrin N, Harte AH, Nungesser R, Quigley N, et al. Uptake of a colorectal cancer screening blood test is higher than of a fecal test offered in clinic: a randomized trial. Cancer Treat Res Commun. 2017;10:27–31.
Bhardwaj M, Gies A, Werner S, Schrotz-King P, Brenner H. Blood-based protein signatures for early detection of colorectal cancer: a systematic review. Clin Transl Gastroenterol. 2017;8:e128.
Wilhelmsen M, Christensen IJ, Rasmussen L, Jørgensen LN, Madsen MR, Vilandt J, et al. Detection of colorectal neoplasia: Combination of eight blood-based, cancer-associated protein biomarkers. Int J Cancer. 2017;140:1436–46.
Rasmussen L, Wilhelmsen M, Christensen IJ, Andersen J, Jørgensen LN, Rasmussen M, et al. Protocol outlines for parts 1 and 2 of the prospective endoscopy III study for the early detection of colorectal cancer: validation of a concept based on blood biomarkers. JMIR Res Protoc. 2016;5:e182.
Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Eur Urol. 2015;67:1142–51.
Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health. 2011;39:72–74.
Rasmussen L, Christensen IJ, Herzog M, Micallef J, Nielsen HJ, Jørgensen LN, et al. Circulating cell-free nucleosomes as biomarkers for early detection of colorectal cancer. Oncotarget. 2018;9:10247–58.
Rho JH, Ladd JJ, Li CI, Potter JD, Zhang Y, Shelley D, et al. Protein and glycomic plasma markers for early detection of adenoma and colon cancer. Gut. 2018;67:473–84.
Jensen SØ, Ørntoft M-BW, Øgaard N, Kristensen H, Rasmussen MH, Bramsen JB, et al. Novel DNA methylation biomarkers show high sensitivity and specificity for blood-based detection of colorectal cancer—a clinical biomarker discovery and validation study. Clin Epigenetics. 2019;11:158.
Imperiale TF, Gruber RN, Stump TE, Emmett TW, Monahan PO. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med. 2019;170:319–29.
Niedermaier T, Balavarca Y, Brenner H. Stage-specific sensitivity of fecal immunochemical tests for detecting colorectal cancer: systematic review and meta-analysis. Am J Gastroenterol. 2020;115:56–69.
Qian J, Tikk K, Werner S, Balavarca Y, Saadati M, Hechtner M, et al. Biomarker discovery study of inflammatory proteins for colorectal cancer early detection demonstrated importance of screening setting validation. J Clin Epidemiol. 2018;104:24–34.
Bhardwaj M, Weigl K, Tikk K, Holland-Letz T, Schrotz-King P, Borchers CH, et al. Multiplex quantitation of 270 plasma protein markers to identify a signature for early detection of colorectal cancer. Eur J Cancer. 2020;127:30–40.
Church TR, Wandell M, Lofton-Day C, Mongin SJ, Burger M, Payne SR, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317–25.
Zhao G, Li H, Yang Z, Wang Z, Xu M, Xiong S, et al. Multiplex methylated DNA testing in plasma with high sensitivity and specificity for colorectal cancer screening. Cancer Med. 2019;8:5619–28.
Amri R, Bordeianou LG, Sylla P, Berger DL. Impact of screening colonoscopy on outcomes in colon cancer surgery. JAMA Surg. 2013;148:747–54.
Brenner H, Jansen L, Ulrich A, Chang-Claude J, Hoffmeister M. Survival of patients with symptom- and screening-detected colorectal cancer. Oncotarget. 2016;7:44695–704.
May FP, Yano EM, Provenzale D, Brunner J, Yu C, Phan J, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17:469–76.
Thomsen MK, Rasmussen M, Njor SH, Mikkelsen EM. Demographic and comorbidity predictors of adherence to diagnostic colonoscopy in the danish colorectal cancer screening program: a nationwide cross-sectional study. Clin Epidemiol. 2018;10:1733–42.
Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–30.
van der Pol Y, Mouliere F. Toward the early detection of cancer by decoding the epigenetic and environmental fingerprints of cell-free DNA. Cancer Cell. 2019;36:350–68.
Nielsen HJ, Christensen IJ, Andersen B, Rasmussen M, Friis-Hansen LJ, Bygott T, et al. Serological biomarkers in triage of FIT-positive subjects? Scand J Gastroenterol. 2017;52:742–4.
Lech Pedersen N, Mertz Petersen M, Ladd JJ, Lampe PD, Bresalier RS, Davis GJ, et al. Development of blood-based biomarker tests for early detection of colorectal neoplasia: Influence of blood collection timing and handling procedures. Clin Chim Acta. 2020;507:39–53.
Acknowledgements
The commitment and excellent work of the research nurses and secretaries at the participating Danish hospitals at the Central Denmark Region and the Capital Region of Denmark are highly appreciated. In addition, the secretaries at the screening centres at Randers and Bornholm Hospitals are thanked for their diligent identification of subjects with FIT-positive and -negative screening results, respectively.
Funding
This study was financially supported by: The Andersen-Isted Fund, The Augustinus Foundation, The Vilhelm Bang Fund, The Beckett Fund, The Inger Bonnén Fund, The Hans and Nora Buchard Fund, CEO Jens Bærentsen, The Walter and O. Kristiane Christensen Fund, The Poul Martin Christiansen Fund, The Aase and Ejnar Danielsen Fund, The Danish Cancer Society, The Erichsen Family Fund, The Knud and Edith Eriksen Fund, The Svend Espersen Fund, The Elna and Jørgen Fagerholt Fund, The Sofus Friis Fund, The Torben and Alice Frimodt Fund, The Eva and Henry Frænkel Fund, The Gangsted Fund, The Thora and Viggo Grove Fund, The Erna Hamilton Fund, The Sven and Ina Hansen Fund, The Hede-Nielsen Family Fund, The Søren and Helene Hempel Fund, The Henrik Henriksen Fund, The Carl and Ellen Hertz Fund, The Elisa and Jørgen Holm Fund, The Humanitarian Foundation, The Ovita Juhl Fund, Foundation Jochum, The KID Fund, The Kornerup Fund, The Linex Fund, The Aage and Johanne Louis-Hansen Fund, The Mid Jutland Newspaper Fund, The Dagmar Marshall Fund, The Muusfeldt Fund, The Børge Nielsen Fund, The Inge and Jørgen Nielsen Fund, The Michael H. Nielsen Fund, The Arvid Nilsson Fund, The Obel Family Fund, The Orient Fund, The Krista and Viggo Petersen Fund, The Willy and Ingeborg Reinhard Fund, The Katrine and Vigo Skovgaard Fund, The Toyota Fund, The Tryg Fund, The Vissing Fund, The Else og Mogens Wedell-Wedellsborg Fund.
Author information
Authors and Affiliations
Contributions
HJN conceived the study and provided the funding. LNJ, JWH, MRM, JV, LM, AK, BA, MR, SG, GJD and IJC helped design the study. LNJ, JWH, MRM, JV, SB, LM, AK, PI, LF, GJD, FM, BA and MR acquired the data. JK, IJC and HJN interpreted the results. JK wrote the draft. All authors revised the manuscript, approved the final version and agreed to be fully accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki, and approved by the Ethics Committee at the Capital Region of Denmark (H-4-2013-050) and the Danish Data Protection Agency (2007-58-0015/HVH-2013-022). All participants signed an informed consent form.
Consent to publish
Not applicable.
Competing interests
SG and GJD are employees of Abbott Laboratories Inc. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kleif, J., Jørgensen, L.N., Hendel, J.W. et al. Early detection of colorectal neoplasia: application of a blood-based serological protein test on subjects undergoing population-based screening. Br J Cancer 126, 1387–1393 (2022). https://doi.org/10.1038/s41416-022-01712-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01712-x