Abstract
Background
Limited accessibility of the tumour precludes longitudinal characterisation for therapy guidance in pancreatic ductal adenocarcinoma (PDAC).
Methods
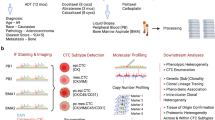
We utilised dielectrophoresis-field flow fractionation (DEP-FFF) to isolate circulating tumour cells (CTCs) in 272 blood draws from 74 PDAC patients (41 localised, 33 metastatic) to non-invasively monitor disease progression.
Results
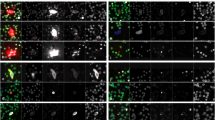
Analysis using multiplex imaging flow cytometry revealed four distinct sub-populations of CTCs: epithelial (E-CTC), mesenchymal (M-CTC), partial epithelial-mesenchymal transition (pEMT-CTC) and stem cell-like (SC-CTC). Overall, CTC detection rate was 76.8% (209/272 draws) and total CTC counts did not correlate with any clinicopathological variables. However, the proportion of pEMT-CTCs (prop-pEMT) was correlated with advanced disease, worse progression-free and overall survival in all patients, and earlier recurrence after resection.
Conclusion
Our results underscore the importance of immunophenotyping and quantifying specific CTC sub-populations in PDAC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics. CA Cancer J. Clin. 69, 7–34 (2019).
Rhim, A. D., Mirek, E. T., Aiello, N. M., Maitra, A., Bailey, J. M., McAllister, F. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Pecot, C. V., Bischoff, F. Z., Mayer, J. A., Wong, K. L., Pham, T., Bottsford-Miller, J. et al. A novel platform for detection of CK+ and CK− CTCs. Cancer Discov. 1, 580–586 (2011).
Siravegna, G., Marsoni, S., Siena, S. & Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 14, 531–548 (2017).
Wei, T., Zhang, X., Zhang, Q., Yang, J., Chen, Q., Wang, J. et al. Vimentin-positive circulating tumor cells as a biomarker for diagnosis and treatment monitoring in patients with pancreatic cancer. Cancer Lett. 452, 237–243 (2019).
Satelli, A., Batth, I., Brownlee, Z., Mitra, A., Zhou, S., Noh, H. et al. EMT circulating tumor cells detected by cell-surface vimentin are associated with prostate cancer progression. Oncotarget 8, 49329–49337 (2017).
Satelli, A., Brownlee, Z., Mitra, A., Meng, Q. H. & Li, S. Circulating tumor cell enumeration with a combination of epithelial cell adhesion molecule- and cell-surface vimentin-based methods for monitoring breast cancer therapeutic response. Clin. Chem. 61, 259–266 (2015).
Chemi, F., Rothwell, D. G., McGranahan, N., Gulati, S., Abbosh, C., Pearce, S. P. et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat. Med. 25, 1534–1539 (2019).
Nagrath, S., Jack, R. M., Sahai, V. & Simeone, D. M. Opportunities and challenges for pancreatic circulating tumor cells. Gastroenterology 151, 412–426 (2016).
Kamyabi, N., Huang, J., Lee, J. J., Bernard, V., Semaan, A., Stephens, B. et al. A microfluidic device for label-free isolation of tumor cell clusters from unprocessed blood samples. Biomicrofluidics 13, 044111 (2019).
DiPardo, B. J., Winograd, P., Court, C. M. & Tomlinson, J. S. Pancreatic cancer circulating tumor cells: applications for personalized oncology. Expert Rev. Mol. Diagn. 18, 809–820 (2018).
Court, C. M., Ankeny, J. S., Sho, S., Hou, S., Li, Q., Hsieh, C. et al. Reality of single circulating tumor cell sequencing for molecular diagnostics in pancreatic cancer. J. Mol. Diagn. 18, 688–696 (2016).
Lapin, M., Tjensvoll, K., Oltedal, S., Javle, M., Smaaland, R., Gilje, B. et al. Single-cell mRNA profiling reveals transcriptional heterogeneity among pancreatic circulating tumour cells. BMC Cancer 17, 390 (2017).
Shim, S., Stemke-Hale, K., Tsimberidou, A. M., Noshari, J., Anderson, T. E. & Gascoyne, P. R. Antibody-independent isolation of circulating tumor cells by continuous-flow dielectrophoresis. Biomicrofluidics 7, 11807 (2013).
Shim, S., Stemke-Hale, K., Noshari, J., Becker, F. F. & Gascoyne, P. R. Dielectrophoresis has broad applicability to marker-free isolation of tumor cells from blood by microfluidic systems. Biomicrofluidics 7, 11808 (2013).
Gascoyne, P. R. & Shim, S. Isolation of circulating tumor cells by dielectrophoresis. Cancers 6, 545–579 (2014).
Balasubramanian, P., Kinders, R. J., Kummar, S., Gupta, V., Hasegawa, D., Menachery, A. et al. Antibody-independent capture of circulating tumor cells of non-epithelial origin with the ApoStream(R) system. PLoS ONE 12, e0175414 (2017).
Allenson, K., Castillo, J., San Lucas, F. A., Scelo, G., Kim, D. U., Bernard, V. et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 28, 741–747 (2017).
Aktas, B., Tewes, M., Fehm, T., Hauch, S., Kimmig, R. & Kasimir-Bauer, S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 11, R46 (2009).
Liu, H., Sun, B., Wang, S., Liu, C., Lu, Y., Li, D. et al. Circulating tumor cells as a biomarker in pancreatic ductal adenocarcinoma. Cell Physiol. Biochem. 42, 373–382 (2017).
Alix-Panabieres, C. & Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 14, 623–631 (2014).
Aiello, N. M. & Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 216, 1016–1026 (2019).
Porter, R. L., Magnus, N. K. C., Thapar, V., Morris, R., Szabolcs, A. Neyaz, A. et al. Epithelial to mesenchymal plasticity and differential response to therapies in pancreatic ductal adenocarcinoma. Proc. Natl Acad. Sci. USA 116, 26835–26845 (2019).
Ting, D. T., Wittner, B. S., Ligorio, M., Vincent Jordan, N., Shah, A. M., Miyamoto, D. T. et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 8, 1905–1918 (2014).
Gemenetzis, G., Groot, V. P., Yu, J., Ding, D., Teinor, J. A., Javed, A. A. et al. Circulating tumor cells dynamics in pancreatic adenocarcinoma correlate with disease status: results of the Prospective CLUSTER Study. Ann. Surg. 268, 408–420 (2018).
Poruk, K. E., Valero, V. 3rd, Saunders, T., Blackford, A. L., Griffin, J. F., Poling, J. et al. Circulating tumor cell phenotype predicts recurrence and survival in pancreatic adenocarcinoma. Ann. Surg. 264, 1073–1081 (2016).
Nieto, M. A., Huang, R. Y., Jackson, R. A. & Thiery, J. P. Emt: 2016. Cell 166, 21–45 (2016).
Aiello, N. M., Maddipati, R., Norgard, R. J., Balli, D., Li, J., Yuan, S. et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev. Cell 45, 681–95 e4 (2018).
Lytle, N. K., Ferguson, L. P., Rajbhandari, N., Gilroy, K., Fox, R. G., Deshpande, A. et al. A multiscale map of the stem cell state in pancreatic adenocarcinoma. Cell 177, 572–86 e22 (2019).
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
A.M., P.A.G., H.A. Study Design: A.M., P.A.G., H.A., A.S., V.B., D.U.K. Provision of clinical samples and information: B.S., G.R.V., M.H.K. Samples processing: A.S., V.B., P.G., D.U.K. Microfluidics device design: P.G., F.A.S.L. Sample isolation: A.S., V.B., P.A.G., D.U.K., N.K. Samples staining and cytometry: A.S., V.B., P.A.G., D.U.K. Data analysis: J.H., F.A.S.L., J.J.L., W.Q., Y.S. P.A.G. Manuscript writing: A.S., V.B. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with standard ethical guidelines approved by the institutional review board (IRB), protocol numbers PA11-0670 and PA15-0014 at MD Anderson Cancer Center, and in accordance with the Declaration of Helsinki. Patients provided their informed consent to participate in this study.
Data availability
All data generated and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
A.M. receives royalties for a pancreatic cancer biomarker test from Cosmos Wisdom Biotechnology, and this financial relationship is managed and monitored by the UTMDACC Conflict of Interest Committee. A.M. is also listed as an inventor on a patent that has been licensed by Johns Hopkins University to Thrive Earlier Detection. The remaining authors declare no competing interests.
Funding information
This research was supported in part by the Cancer Prevention and Research Institute of Texas (CPRIT) (No. RP160517), NCI P50 CA221707, U01 CA196403 and U01 CA200468 to A.M. N.K. was supported by the CPRIT Research Training Program (No. RP170067). V.B. was supported by the CPRIT Research Training Program (Nos. RP140106 and RP170067) and NCI (Nos. T32CA217789-03 and U54CA096297). J.J.L. was supported by the National Institutes of Health (NIH) (No. T32CA009599). A.S. was supported by the German Research Foundation (SE-2616/2-1). D.U.K. was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1C1B5086234).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Semaan, A., Bernard, V., Kim, D.U. et al. Characterisation of circulating tumour cell phenotypes identifies a partial-EMT sub-population for clinical stratification of pancreatic cancer. Br J Cancer 124, 1970–1977 (2021). https://doi.org/10.1038/s41416-021-01350-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01350-9
This article is cited by
-
Liquid biopsy techniques and pancreatic cancer: diagnosis, monitoring, and evaluation
Molecular Cancer (2023)
-
Feasibility of mass cytometry proteomic characterisation of circulating tumour cells in head and neck squamous cell carcinoma for deep phenotyping
British Journal of Cancer (2023)
-
Reactivation of embryonic genetic programs in tissue regeneration and disease
Nature Genetics (2023)
-
Epigenetic control of pancreatic cancer metastasis
Cancer and Metastasis Reviews (2023)
-
Metastasis Prevention: Focus on Metastatic Circulating Tumor Cells
Molecular Diagnosis & Therapy (2021)