Abstract
Background
Maximal effort cytoreductive surgery is associated with improved outcomes in advanced high-grade serous ovarian cancer (HGSOC). However, despite complete gross resection (CGR), there is a percentage of patients who will relapse and die early. The aim of this study is to identify potential candidate biomarkers to help personalise surgical radicality.
Methods
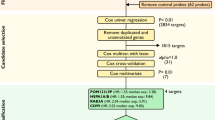
136 advanced HGSOC cases who underwent CGR were identified from three public transcriptomic datasets. Candidate prognostic biomarkers were discovered in this cohort by Cox regression analysis, and further validated by targeted RNA-sequencing in HGSOC cases from Imperial College Healthcare NHS Trust (n = 59), and a public dataset. Gene set enrichment analysis was performed to understand the biological significance of the candidate biomarker.
Results
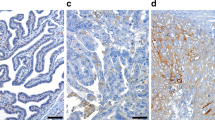
We identified ALG5 as a prognostic biomarker for early tumour progression in advanced HGSOC despite CGR (HR = 2.42, 95% CI (1.57–3.75), p < 0.0001). The prognostic value of this new candidate biomarker was additionally confirmed in two independent datasets (HR = 1.60, 95% CI (1.03–2.49), p = 0.0368; HR = 3.08, 95% CI (1.07–8.81), p = 0.0365). Mechanistically, the oxidative phosphorylation was demonstrated as a potential biological pathway of ALG5-high expression in patients with early relapse (p < 0.001).
Conclusion
ALG5 has been identified as an independent prognostic biomarker for poor prognosis in advanced HGSOC patients despite CGR. This sets a promising platform for biomarker combinations and further validations towards future personalised surgical care.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hu, M., Fu, X., Si, Z., Li, C., Sun, J., Du, X. et al. Identification of differently expressed genes associated with prognosis and growth in colon adenocarcinoma based on integrated bioinformatics analysis. Front Genet10, 1245 (2019).
Caputo R., Cianniello D., Giordano A., Piezzo M., Riemma M., Trovo M., et al. Gene expression assay in the management of early breast cancer. Curr Med Chem.27, 2826–2839 (2019).
Fotopoulou, C., Hall, M., Cruickshank, D., Gabra, H., Ganesan, R., Hughes, C. et al. British Gynaecological Cancer Society (BGCS) epithelial ovarian/fallopian tube/primary peritoneal cancer guidelines: recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol.213, 123–139 (2017).
Ray-Coquard, I., Pautier, P., Pignata, S., Perol, D., Gonzalez-Martin, A., Berger, R. et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N. Engl. J. Med.381, 2416–2428 (2019).
Coleman, R. L., Fleming, G. F., Brady, M. F., Swisher, E. M., Steffensen, K. D., Friedlander, M. et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med.381, 2403–2415 (2019).
Fotopoulou, C. How to predict treatment failure in frail patients with advanced epithelial ovarian cancer: strategies to personalize surgical effort. J. Gynecol. Oncol.31, e26 (2020).
Hall, M., Savvatis, K., Nixon, K., Kyrgiou, M., Hariharan, K., Padwick, M. et al. Maximal-effort cytoreductive surgery for ovarian cancer patients with a high tumor burden: variations in practice and impact on outcome. Ann. Surgical Oncol.26, 2943–2951 (2019).
Harter, P., Sehouli, J., Lorusso, D., Reuss, A., Vergote, I., Marth, C. et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N. Engl. J. Med.380, 822–832 (2019).
du Bois, A., Reuss, A., Pujade-Lauraine, E., Harter, P., Ray-Coquard, I. & Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer115, 1234–1244 (2009).
Heitz, F., Kommoss, S., Tourani, R., Grandelis, A., Uppendahl, L., Aliferis, C. et al. Dilution of molecular-pathologic gene signatures by medically associated factors might prevent prediction of resection status after debulking surgery in patients with advanced ovarian cancer. Clin. Cancer Res.26, 213–219 (2020).
Phelps, D. L., Borley, J. V., Flower, K. J., Dina, R., Darb-Esfahani, S., Braicu, I. et al. Methylation of MYLK3 gene promoter region: a biomarker to stratify surgical care in ovarian cancer in a multicentre study. Br. J. Cancer116, 1287–1293 (2017).
Lu, H., Arshad, M., Thornton, A., Avesani, G., Cunnea, P., Curry, E. et al. A mathematical-descriptor of tumor-mesoscopic-structure from computed-tomography images annotates prognostic- and molecular-phenotypes of epithelial ovarian cancer. Nat. Commun.10, 764 (2019).
Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature474, 609–615 (2011).
Tothill, R. W., Tinker, A. V., George, J., Brown, R., Fox, S. B., Lade, S. et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res.14, 5198–5208 (2008).
Denkert, C., Budczies, J., Darb-Esfahani, S., Gyorffy, B., Sehouli, J., Konsgen, D. et al. A prognostic gene expression index in ovarian cancer—validation across different independent data sets. J. Pathol.218, 273–280 (2009).
Ferriss, J. S., Kim, Y., Duska, L., Birrer, M., Levine, D. A., Moskaluk, C. et al. Multi-gene expression predictors of single drug responses to adjuvant chemotherapy in ovarian carcinoma: predicting platinum resistance. PLoS ONE7, e30550 (2012).
Rustin, G. J., van der Burg, M. E., Griffin, C. L., Guthrie, D., Lamont, A., Jayson, G. C. et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet376, 1155–1163 (2010).
Fotopoulou, C., Concin, N., Planchamp, F., Morice, P., Vergote, I., du Bois, A. et al. Quality indicators for advanced ovarian cancer surgery from the European Society of Gynaecological Oncology (ESGO): 2020 update. Int J. Gynecol. Cancer30, 436–440 (2020).
Prat, J., Oncology, F. C. O. G., Belhadj, H., Berek, J., Bermudez, A., Bhatla, N. et al. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J. Gynaecol. Obstet.124, 1–5 (2014).
McShane, L. M., Altman, D. G., Sauerbrei, W., Taube, S. E., Gion, M. & Clark, G. M. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res. Treat.100, 229–235 (2006).
Subramanian, A., Tamayo, P., Mootha, V. K., Mukherjee, S., Ebert, B. L. & Gillette, M. A. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA102, 15545–15550 (2005).
Heesen, S., Lehle, L., Weissmann, A. & Aebi, M. Isolation of the ALG5 locus encoding the UDP-glucose:dolichyl-phosphate glucosyltransferase from Saccharomyces cerevisiae. Eur. J. Biochem. 224, 71–79 (1994).
Larkin, A., Chang, M. M., Whitworth, G. E. & Imperiali, B. Biochemical evidence for an alternate pathway in N-linked glycoprotein biosynthesis. Nat. Chem. Biol.9, 367–373 (2013).
Matassa, D. S., Amoroso, M. R., Lu, H., Avolio, R., Arzeni, D., Procaccini, C. et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ.23, 1542–1554 (2016).
Hernlund, E., Hjerpe, E., Avall-Lundqvist, E. & Shoshan, M. Ovarian carcinoma cells with low levels of beta-F1-ATPase are sensitive to combined platinum and 2-deoxy-D-glucose treatment. Mol. Cancer Ther.8, 1916–1923 (2009).
Ricci, F., Brunelli, L., Affatato, R., Chila, R., Verza, M., Indraccolo, S. et al. Overcoming platinum-acquired resistance in ovarian cancer patient-derived xenografts. Ther. Adv. Med. Oncol.11, 1758835919839543 (2019).
Fotopoulou, C. ASO author reflections: value of cytoreduction in extensive ovarian cancer-does surgical effort still matter? Ann. Surgical Oncol.26, 786–787 (2019).
Weinberg, S. E. & Chandel, N. S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol.11, 9–15 (2015).
Ashton, T. M., McKenna, W. G., Kunz-Schughart, L. A. & Higgins, G. S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res.24, 2482–2490 (2018).
Nayak A. P., Kapur A., Barroilhet L., Patankar M. S. Oxidative phosphorylation: a target for novel therapeutic strategies against ovarian cancer. Cancers (Basel). 10, 337 (2018).
Dar, S., Chhina, J., Mert, I., Chitale, D., Buekers, T., Kaur, H. et al. Bioenergetic adaptations in chemoresistant ovarian cancer cells. Sci. Rep.7, 8760 (2017).
Pasto, A., Bellio, C., Pilotto, G., Ciminale, V., Silic-Benussi, M., Guzzo, G. et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget5, 4305–4319 (2014).
Anderson, A. S., Roberts, P. C., Frisard, M. I., Hulver, M. W. & Schmelz, E. M. Ovarian tumor-initiating cells display a flexible metabolism. Exp. Cell Res.328, 44–57 (2014).
Acknowledgements
Tissue samples were provided by the Imperial College Healthcare NHS Trust Tissue Bank. Other investigators may have received samples from these same tissues. We acknowledge Ms Naina Patel, Ms Nona Rama and Dr. Ed Curry for help and suggestions on tumour collection, study design and data analysis.
Author information
Authors and Affiliations
Contributions
C.F. and H.L. conceived and designed this study. K.N. and N.R. collected the clinical annotations. P.C. prepared the tumour samples. H.L. collected the RNA-sequencing data and performed bioinformatics analysis. H.L., P.C., K.N., N.R., E.A. and C.F. contributed to the interpretation of data. H.L., P.C. and C.F. wrote the manuscript. All authors revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Frozen tumour specimens were provided by the Imperial College Healthcare NHS Trust Tissue Bank after full informed patient consent. The ethical approval was obtained from the Hammersmith and Queen Charlotte’s & Chelsea Research Ethics Committee approval 05/QO406/178. The procedures involved human participants were done in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to publish
This manuscript does not contain any individual patient data.
Data availability
The RNA-sequencing data and clinical annotations are accessible in Mendeley Data with the identifier https://doi.org/10.17632/kw736vjpsd.1.
Competing interests
The authors declare no competing interests.
Funding information
This study is supported by the National Institute for Health Research (NIHR), Imperial Biomedical Research Centre (BRC), the Imperial College Experimental Cancer Medicine Centre (ECMC), and Cancer Research UK. C.F., P.C., K.N. and N.R. acknowledge funding from Imperial Health Charity, Myrovlytis Trust and Imperial Private Healthcare. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, H., Cunnea, P., Nixon, K. et al. Discovery of a biomarker candidate for surgical stratification in high-grade serous ovarian cancer. Br J Cancer 124, 1286–1293 (2021). https://doi.org/10.1038/s41416-020-01252-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-01252-2
This article is cited by
-
An integrated machine learning-based model for joint diagnosis of ovarian cancer with multiple test indicators
Journal of Ovarian Research (2024)
-
Neoadjuvant chemotherapy reduces the levels of HMGB1 and E-cadherin in patients with breast cancer
Scientific Reports (2023)
-
Validation analysis of the novel imaging-based prognostic radiomic signature in patients undergoing primary surgery for advanced high-grade serous ovarian cancer (HGSOC)
British Journal of Cancer (2022)