Abstract
Background
Human papillomavirus (HPV) is a necessary cause of cervical cancer, although some invasive cervical cancers may test negative by HPV PCR. We previously requested all invasive cervical cancers in Sweden during 10 years and subjected them to PCR. We also optimised methods for deep sequencing of formalin-fixed paraffin-embedded samples.
Methods
Using Novaseq 6000, we simultaneously sequenced total DNA and cDNA from 392 HPV PCR-negative cervical cancers. Non-human reads were queried against all known HPVs. The complete database now contains PCR and/or deep sequencing data on 2850 invasive cervical cancers.
Results
HPV sequences were detected in 169/392 of HPV PCR-negative cervical cancers. Overall, 30 different HPV types were detected, but only 5 types were present in proportions above 3% of cancers. More than 92% of tumours were HPV-positive in PCR and/or sequencing (95% confidence interval: 91.1–93.1%). Exploring possible reasons for failure to previously detect HPV suggest that more sensitive type-specific PCRs for HPV 31, 33, 45 and 73 targeting retained regions of HPV would have detected most of these (117/392).
Conclusions
Unbiased deep sequencing provides comprehensive data on HPV types in cervical cancers and appears to be an important tool for quality assurance of HPV screening.
Similar content being viewed by others
Background
While human papillomavirus (HPV) is a necessary cause for cervical cancer, several authors have reported a proportion of invasive cervical cancers as being HPV negative. The range of HPV negativity varies between studies: in some cervical cancer cohorts, just 0.03% cervical tumours have no HPV detected while others report up to 15% cervical cancers being HPV negative.1,2,3
Possible explanations for not finding HPV among cervical cancers include: cancers independent of HPV (rare true negatives), loss of HPV genomes, cancers caused by HPV types not tested for, misclassification of cancers (e.g. metastasis from other tumours or cancers of corpus uteri misclassified as cervical) and failure of HPV detection methods.1,2,3,4
To optimise cervical screening, the risk of false negativity in HPV screening needs to be minimised at all stages. HPV-test negative cervical cancers may constitute a biologically distinct subgroup, associated with symptomatic detection, late-stage diagnosis and worse prognosis5 that may need different targeted therapeutic strategies.6
Common methods to detect HPV are based on PCR and probe hybridisation, usually targeting the L1 gene, which is the most conserved gene within the HPV genome.7 These PCR methods are both efficient and sensitive but are unable to detect HPVs that do not bind specifically to designed primers and probes. An HPV genotype that diverges in its genomic sequence from the sequences designed for primers/probe may escape amplification and/or hybridisation, and therefore remain undetected.8,9,10 HPV detection failure can be readily circumvented by subjecting specimens to unbiased high throughput sequencing of the total nucleic acids of the sample, as HPV can then be detected without prior knowledge/assumptions of which genotype-specific sequences may be present.8,11,12 Furthermore, if cDNA is sequenced, the data will show if there is viral transcriptional activity, which is typically essential for both initiation and maintenance of the malignant phenotype.
We thus aimed to assess whether unbiased deep sequencing might detect HPV among cervical cancer specimens testing HPV negative by PCR.
Methods
Sample collection
HPV genotyping was previously performed based on L1 amplification and consequent probe hybridisation on formalin-fixed paraffin-embedded (FFPE) blocks from all cervical cancers occurring during a 10-year period (2002–2011) in Sweden.13 In case of HPV negativity, the slide from each case-block was re-reviewed by a pathologist to confirm presence of tissue representative of cervical cancer. FFPE cervical blocks were thereafter subjected to real-time PCR (rt-PCR) for HPV 16 and 18 targeting E6 and E7 genes of the HPV genome. Out of the 2850 cervical cases, we reported that almost 14% (394/2850) were HPV negative by PCR.13
For this study, we included all HPV negative FFPE blocks (n = 394) together with 59 randomly selected HPV positive blocks to be used as positive controls, to assess the overall presence of HPV using an unbiased (i.e. not based on PCR) approach with deep sequencing. As negative controls, we selected all corresponding empty paraffin blocks, “blank blocks”, that had been sectioned in-between each cervical cancer FFPE block (n = 453).
A total of 34/59 HPV positive blocks and 92/394 cancers negative by HPV PCR had been already sequenced as part of a methods optimisation paper.14 To obtain a comprehensive result of the HPV status in invasive cervical cancers in Sweden during a 10-year period, the previous PCR data and the previous sequencing data were combined with the new sequencing performed here.
Deep sequencing
All FFPE blocks (n = 906) were extracted as previously described.15 Cervical FFPE blocks were divided randomly into 7 groups for sequencing. The blank blocks corresponding to the cases in each group, were divided and pooled into 2 negative controls. As a result, each group contained approximately 65 cervical blocks (HPV positive and negative cervical blocks) and 2 negative controls (containing each of these controls, a pool with extracted material from half of the empty paraffin blocks corresponding to the FFPE cases within that group).
For each sample, 8 μl of extracted material was reverse-transcribed, indexed and rRNA-depleted following the SMARTer® Stranded Total RNA-Seq Kit v2 - Pico Input Mammalian library preparation guide (Takara, US), omitting the fragmentation step. The libraries were validated, normalised to 2 nM and pooled according to the 7 groups division before sequencing. RNA seq was performed on NovaSeq 6000 system (Illumina, USA) at 2 × 150 bp, in seven different runs (one run per group), aiming for 30 M paired end reads/sample.
Bioinformatics
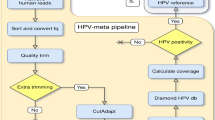
Indices, included in the Illumina adaptors, were used to assign raw sequence reads obtained from the NovaSeq 6000 (Illumina) platform to the originating samples. Reads were quality- and adaptor-trimmed with Trimmomatic16 using default parameters and 18 bp as minimal read length. The first 3 nucleotides from every R2 read were trimmed, as advised within the SMARTer pico kit used for library preparation, and thereafter high-quality paired reads were screened against the human reference genome GRCh38 using NextGenMap v.0.5.2.17 Reads were filtered out if they aligned with >95 % identity over 75 % of their length to the human genome. Non-human reads were queried against all HPV protein sequences included in the PaVE database (Papillomavirus Episteme, accessed on 28 July 2019, including all protein sequences from HPV reference and non-reference genomes), using the open source software Diamond18 blastx with default parameters and –top 1. Samples were considered positive for HPV (cut-off) if a minimum of 10 reads were detected for any HPV type with at least 90 % identity, and a coverage of >10% of that HPV genotype (approx. 790 bp) was present.
Analysis of the MGP region
Cervical samples classified as positive with deep sequencing which presented HPV genotypes that should have been but were not previously detected by PCR, were subjected to further analysis to elucidate reasons for the false negativity. We analysed the genomic sequence and coverage of HPV reads within the MGP region in the L1 gene, which is the PCR region targeted for genotyping using a modified general primer (MGP) system,19 as well as possible mismatches with primers and probes used in the genotyping method.
Non-human reads were aligned to an HPV database comprising all HPV genomes officially established by the International HPV Reference Center (n = 221), https://www.hpvcenter.se, accessed on 2020–01–20), together with complete genome sequences from HPV types that are not officially established yet (n = 222, https://pave.niaid.nih.gov, accessed on 2020–01–20), using NextGenMap,17 and >90% identity over 75% of read length as selected parameters for alignment. HPV-aligned reads from each sample were thereafter subjected to visual inspection using Integrative Genomics Viewer to confirm mapping of reads within the corresponding genotype, assess coverage and SNPs existence.
Confirmation of presence of HPV types by rt-PCR
Rt-PCR was used to confirm presence/absence of HPV types in case of discrepancies between PCR-based genotyping and deep sequencing or when a blank block had tested positive. In the cancer cases where this happened, all cervical tumour samples, together with all individual empty paraffin blank blocks within the sequencing run, were subjected to rt-PCR. The rt-PCR was performed at 20 µL reaction volume containing 50–100 ng genomic DNA, 1× TaqMan Universal PCR Master Mix (Applied Biosystems) including 0.5 µM of each forward and reverse primer and 0.25 µM HPV MGB-probe. Water was used as non-template control in each run. The PCR reaction was carried out in ABI 7300 Real-time PCR System, using the Software version 2.0.5 (Applied Biosystems), with the following temperature settings: 10 min at 95 °C, followed by 45 cycles at 95 °C for 15 s and 60 °C for 1 min. The threshold was set to 0.1 ∆Rn (Rn is the fluorescence of the reporter dye divided by the fluorescence of the passive reference. For ∆Rn the baseline fluorescence has been subtracted).
When there was a disagreement in genotyping results between the PCR based method and sequencing, the rt-PCR mixtures contained a total of 20 µL: 5 µL sample and 1× of TaqMan Fast Advanced Master Mix (Applied Biosystems) including 0.5 µM of each primer and 0.25 µM HPV probe. Water was used as non-template control in each run. The PCR analyses were carried out in QuantStudio 3, using the QuantStudio Design & Analysis Software v.1.4.3 (Applied Biosystems), with the following temperature settings: 20 min at 95 °C, followed by 45 cycles at 95 °C for 1 min and 60 °C for 20 s.
Results
Among all the cases of invasive cervical cancer in Sweden over a 10-year period, we could perform HPV genotyping with PCR and/or deep sequencing for 2850 cases. All FFPE tumour blocks and their corresponding blank paraffin blocks, were first subjected to HPV genotyping. If HPV negative (n = 483), the FFPE blocks were then subjected to HPV 16 and 18 rt-PCR targeting E6 and E7 genes of the HPV genome. In case still being HPV negative (n = 394), the samples were then deep sequenced following an unbiased approach, not based on PCR. A consort diagram can be found in Supplementary Fig. 1.
The median age at cancer diagnosis of the HPV negative cervical cases (n = 394) was 62.5 years (range 24–95 years); most women were diagnosed at age 60 or above. Sixty-five percent of the HPV negative cervical cancers were squamous cell carcinoma, 23 % were adenocarcinoma and 12 % were less common histological types, such as adenosquamous cell carcinoma and other rare types. Compared to cases positive for HPV, HPV negative cases were more likely to be diagnosed at older age and more advanced stage (Table 1).
There were two specimens that had been HPV-negative by PCR but, had too little material available for sequencing and thus only 392/394 HPV-negative cancers were sequenced. We also sequenced 59 HPV PCR-positive cervical cancers, selected at random, for comparability of methods.
The NovaSeq sequencing generated high-quality sequencing data, with a median of 2898 million paired reads per run and 30 million paired reads per sample. Most reads (median 96%) were classified as human sequences. We detected HPV sequences in 54/59 HPV PCR-positive cancers, with total concordance regarding the HPV type detected in 46/59 (77.97%) specimens (Table 2), partial concordance in 3/59 specimens (5.08%), and HPV positivity but with a different genotype than detected by PCR in 5/59 (8.47%) specimens (Table 2). Five specimens were HPV negative by sequencing.
HPV sequences were detected in 169/392 HPV PCR-negative specimens, with all samples presenting a single infection. HPV 33, 73, 31 and 45 were detected in 117/392 of all HPV PCR negative cervical cancer cases (58/392, 23/392, 19/392 and 17/392 specimens, respectively) (Table 3).
Combining the deep sequencing results from this paper with previously reported results13,14 to provide a comprehensive overview of the presence of specific HPV types in cervical cancers in Sweden found some type of HPV in 92.17% (2625/2848) of all cervical cancers cases (Table 4). There was a total of 30 different HPV types detected but only five types (HPV 16/18/45/33 or 31) were found in >3% of cancers (Table 4).
Analysis of the MGP region
One hundred and sixty-four cervical cancers HPV-negative by PCR (164/169, 97.04%) together with 5/59 (8.47%) previously classified as positive with HPV PCR, presented HPV genotypes that should have been previously detected by the PCR-based method but were not. Analysis of the sequencing reads present in the MGP region in the L1 gene (the PCR region targeted for genotyping) revealed that 109/169 cancers had reads covering the MGP region, with 72/109 samples showing identical sequence to the corresponding HPV genotype. Samples presenting with sequences that differed from the PCR-targeted region (37/109) did not show more than 2-point substitutions when comparing the reference genotype sequence comprising the forward primer, probe or reverse primer binding regions. Most of them (23/37) had only a single substitution in one of the three binding regions (Table 5).
Confirmation of presence of HPV types by rt-PCR
We sequenced 14 pools of blank blocks which comprised all empty paraffin blocks that had been sectioned in-between each cervical cancer FFPE block. Twelve pools showed no traces of HPV, 1 pool revealed presence of HPV 85 (a genotype that had not been detected in any of the case specimens of the study) and 1 pool contained 51 reads of HPV 33. Among the 70 samples sequenced within the same run as the HPV 33 positive blank block, there were 16 HPV 33-positive samples.
We, therefore, subjected all 70 cervical tumours and all 70 blank blocks in this particular run to type-specific real-time PCR targeting HPV 33. Primers and probes for HPV 33 have been previously described.20 The Rt-PCR confirmed HPV 33 presence in all reported HPV 33 positive samples (16/16) and also found it in one cervical tumour where it had not been found by sequencing. All the individual empty paraffin blank blocks were negative for HPV 33 by rt-PCR.
In the analyses of the five samples where the PCR and the sequencing had found different genotypes, the rt-PCR found the same HPV genotype as found by the sequencing in all 5 cases.
Discussion
We find that unbiased (not based on methods requiring prior knowledge of the sequences aimed to be detected) deep sequencing of FFPE specimens of invasive cervical cancers provides improved accuracy and detectability, detecting HPV in 169/392 cancers that had been negative by HPV PCR.
Accurate detection of HPV is essential, as false negativity may translate into failure in the HPV-based cervical screening program. All specimens that were included in this study were confirmed by surgical staging, histopathological re-review, and specimen adequacy assessment (beta-globin detection) to rule out the possibility of HPV negativity being due to misclassification of the tumour and/or specimen inadequacy.13,21 While a few of the HPV types detected with deep sequencing (HPV 32, 34 and 38) were not tested for with the PCR detection method used, most of the samples (164/169) revealed HPV types, that should have been detected with the PCR based method. Further analysis of the MGP region (region targeted by PCR primers and probe) revealed that many of the PCR HPV negative cervical cancers (60/169) had no sequences present from the L1 region in the samples, which might explain why a PCR method targeting L1 failed to detect the HPV. Thirty-seven samples showed sequence variability in the sequences targeted by primers or probes, although with not more than 2 substitutions in either one primer and/or probe. For the remaining 72 cancers, we cannot explain why the HPV was not detected before. Our PCR method has a sensitivity of 50 international units of HPV 16 and HPV 18 and 500 genome equivalents for the other oncogenic HPV types,22 suggesting that the difference may be attributable to the higher sensitivity of the sequencing.
The majority of the HPV PCR negative samples that showed HPV presence after being sequenced contained high-risk or probable high-risk HPV types (161/169, 95.27%), and only 8/169 (4.73% specimens showed presence of low-risk HPV types 32 (n = 1), 34 (n = 3), 38 (n = 1), 42 (n = 2), and 70 (n = 1), in line with the fact that low-risk HPV types are only occasionally found in cervical cancer.
We report that there are still 223/2850 (7.82%) cervical cancers where HPV was not detected. A previous meta-analysis including 30,848 invasive cervical cancers worldwide reported an increased overall HPV prevalence from 85.9% in studies published from 1990 to 1999 to 92.9% in studies published from 2006 to 2010.4 Similar percentages of HPV-negativity in invasive cervical cancers are reported by other authors (7–11%).1,4,21
HPV negativity was diagnosed based on both PCR and next-generation sequencing, and not on immunohistochemistry (cytokeratin 5, pan cytokeratin, protein 63, P16 and P53). In this study, we had pre-specified a cut-off of 10 HPV reads and at least 10% HPV genome coverage for a sample to be considered as HPV positive, to avoid possible false positivity. There were some samples that presented a weak signal of HPV (<10 reads and/or <10% of HPV genome coverage according to our definition). Further studies are needed to elucidate the reproducibility and significance of these.
Factors as duration of tissue storage, type of cancer histology, and stage of cervical cancer are reported to correlate with the detectability of HPV in cervical tumours.23,24,25,26 The HPV prevalence was indeed very high (96.42%) in stage Ia stage, compared to 84.13% in III+ stage, in line with previous publications suggesting that HPV expression can be lost as the stage of the cervical cancer is progressing.23
One retrospective study reported that the duration of tissue storage had a statistically significant impact on HPV detection (p < 0.005), with cases from the last 30 years showing around 24% HPV negativity and older cases (stored for >60 years) presenting a significantly lower HPV detection rate, with >50% HPV negativity.25 Our study comprised tumour blocks obtained during a 10-year period and therefore, no differences are expected among the HPV detection rate due to the different storage time among them.
It is also known that HPV negativity is more common in several cervical cancer histologies. While squamous cancers and adenocarcinomas in situ are hardly ever HPV negative,26 adenosquamous tumours show an HPV prevalence of about 86%24 and the HPV prevalence among adenocarcinomas varies between the subtypes.25 The present study showed an HPV prevalence of 95.40% for squamous cell carcinomas, 84.28% for adenocarcinomas, 82.35% for adenosquamous carcinomas and 76.09% for other rare invasive cervical cancers, again in line with previous literature.
As reported previously, we chose to perform reverse transcription of mRNAs without DNAse treatment to obtain a maximally sensitive combination of cDNA and some genomic DNA as well.14 We detected reads with known HPV viral splice junctions in 81/169 (48%) of the HPV-positive cancer specimens (data not shown), showing that at least a part of these FFPE specimens had some viral mRNA present.14
Despite the fact that HPV negative cancers do exist, HPV screening is by far the best option to eliminate cervical cancer. Data from randomised controlled trials are very clear on the very low cervical cancer risk after an HPV-negative screening test.27 The studies in our paper are well in line with the findings that HPV screening has higher sensitivity than any other alternative screening method.
In summary, we report that when cervical cancers are tested by both PCR and deep sequencing, HPV sequences exist in >92% cervical cancer specimens. Type-specific PCRs targeting retained regions of the HPV genome of the most common HPV types might be useful for optimal sensitivity in HPV screening. In addition, we suggest that deep sequencing of apparently HPV-negative cervical cancers is useful for quality assurance in HPV screening and detection of HPV infections undetected by HPV PCR methods.
References
Tjalma, W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis. Obgyn 10, 107–113 (2018).
Walboomers, J. M., Jacobs, M. V., Manos, M. M., Bosch, F. X., Kummer, J. A., Shah, K. V. et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189, 12–19 (1999).
Cancer Genome Atlas Research N, Albert Einstein College of M, Analytical Biological S, Barretos Cancer H, Baylor College of M, Beckman Research Institute of City of H. et al. Integrated genomic and molecular characterization of cervical cancer. Nature 543, 378–384 (2017).
Li, N., Franceschi, S., Howell-Jones, R., Snijders, P. J. & Clifford, G. M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J. Cancer 128, 927–935 (2011).
Lei, J., Ploner, A., Lagheden, C., Eklund, C., Nordqvist Kleppe, S., Andrae, B. et al. High-risk human papillomavirus status and prognosis in invasive cervical cancer: A nationwide cohort study. PLoS Med 15, e1002666 (2018).
Banister, C. E., Liu, C., Pirisi, L., Creek, K. E. & Buckhaults, P. J. Identification and characterization of HPV-independent cervical cancers. Oncotarget 8, 13375–13386 (2017).
Abreu, A. L., Souza, R. P., Gimenes, F. & Consolaro, M. E. A review of methods for detect human papillomavirus infection. Virol. J. 9, 262 (2012).
Arroyo Muhr, L. S., Bzhalava, D., Lagheden, C., Eklund, C., Johansson, H., Forslund, O. et al. Does human papillomavirus-negative condylomata exist? Virology 485, 283–288 (2015).
Arroyo, L. S., Smelov, V., Bzhalava, D., Eklund, C., Hultin, E. & Dillner, J. Next generation sequencing for human papillomavirus genotyping. J. Clin. Virol. 58, 437–442 (2013).
Arroyo Muhr, L. S., Hultin, E., Bzhalava, D., Eklund, C., Lagheden, C., Ekstrom, J. et al. Human papillomavirus type 197 is commonly present in skin tumors. Int J. Cancer 136, 2546–2555 (2015).
Bzhalava, D., Johansson, H., Ekstrom, J., Faust, H., Moller, B., Eklund, C. et al. Unbiased approach for virus detection in skin lesions. PLoS ONE 8, e65953 (2013).
Arroyo Muhr, L. S., Bzhalava, Z., Hortlund, M., Lagheden, C., Nordqvist Kleppe, S., Bzhalava, D. et al. Viruses in cancers among the immunosuppressed. Int J. Cancer 141, 2498–2504 (2017).
Lagheden, C., Eklund, C., Lamin, H., Kleppe, S. N., Lei, J., Elfstrom, K. M. et al. Nationwide comprehensive human papillomavirus (HPV) genotyping of invasive cervical cancer. Br. J. Cancer 118, 1377–1381 (2018).
Arroyo Muhr, L. S., Lagheden, C., Eklund, C., Lei, J., Nordqvist-Kleppe, S., Sparen, P. et al. Sequencing detects human papillomavirus in some apparently HPV-negative invasive cervical cancers. J. Gen. Virol. 101, 265–270 (2020).
Lagheden, C., Eklund, C., Kleppe, S. N., Unger, E. R., Dillner, J. & Sundstrom, K. Validation of a standardized extraction method for formalin-fixed paraffin-embedded tissue samples. J. Clin. Virol. 80, 36–39 (2016).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Sedlazeck, F. J., Rescheneder, P. & von Haeseler, A. NextGenMap: fast and accurate read mapping in highly polymorphic genomes. Bioinformatics 29, 2790–2791 (2013).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Soderlund-Strand, A., Carlson, J. & Dillner, J. Modified general primer PCR system for sensitive detection of multiple types of oncogenic human papillomavirus. J. Clin. Microbiol 47, 541–546 (2009).
Lindh, M., Gorander, S., Andersson, E., Horal, P., Mattsby-Balzer, I. & Ryd, W. Real-time Taqman PCR targeting 14 human papilloma virus types. J. Clin. Virol. 40, 321–324 (2007).
Petry, K. U., Liebrich, C., Luyten, A., Zander, M. & Iftner, T. Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res 4, 85–89 (2017).
Eklund, C., Forslund, O., Wallin, K. L. & Dillner, J. Continuing global improvement in human papillomavirus DNA genotyping services: The 2013 and 2014 HPV LabNet international proficiency studies. J. Clin. Virol. 101, 74–85 (2018).
Molijn, A., Jenkins, D., Chen, W., Zhang, X., Pirog, E., Enqi, W. et al. The complex relationship between human papillomavirus and cervical adenocarcinoma. Int. J. Cancer 138, 409–416 (2016).
Holl, K., Nowakowski, A. M., Powell, N., McCluggage, W. G., Pirog, E. C., De Souza, S. C. et al. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a European multinational epidemiological study. Int. J. Cancer 137, 2858–2868 (2015).
Pirog, E. C., Lloveras, B., Molijn, A., Tous, S., Guimera, N., Alejo, M. et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod. Pathol. 27, 1559–1567 (2014).
Pirog, E. C. Cervical Adenocarcinoma Diagnosis of Human Papillomavirus-Positive and Human Papillomavirus-Negative Tumors. Arch. Pathol. Lab Med 141, 1653–1667 (2017).
Arbyn, M., Ronco, G., Anttila, A., Meijer, C. J., Poljak, M., Ogilvie, G. et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 30, F88–F99 (2012).
Acknowledgements
The authors would like to acknowledge support from Science for Life Laboratory, the National Genomics Infrastructure, NGI, and Uppmax for assistance in massive parallel sequencing and computational infrastructure. We thank all members of the Advancing Cervical Cancer Eradication Strategies group (ACCES.nu) for many inspiring scientific discussions, and Sadaf Sakina Hassan and Mehran Ghaderi for excellent technical assistance with rt-PCR.
Author information
Authors and Affiliations
Contributions
S.N-K., J.D., P.S. and K.S. have collected all the samples. L.S.A.M., C.L. and C.E., performed laboratory work and planning of the sequencing. L.S.A.M. performed all bioinformatical analyses. L.S.A.M., C.L., J.L, and C.E. performed data interpretation. L.S.A.M. and J.D. designed the study and J.D. and K.S. provided supervision. L.S.A.M. and J.D. drafted and coordinated the writing of the paper. All authors have critically revised the paper for important intellectual content and approved the final version for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was granted by the Regional Ethical Review Board of Stockholm, Sweden (EPN-Dnr: 2012/1028/32). The Regional Ethical Review Board determined that, due to the population-based nature of the study, informed consent from study participants was not required (EPN-Dnr: 2011/1026-31/4).
Consent to publish
Not applicable.
Data availability
All the aligned, non-human sequences are available at the Sequence Read Archive (SRA) within the bio-project ID PRJNA563802.
Competing interests
The authors declare no competing interests.
Funding information
Funding was obtained from the Swedish Foundation for Strategic Research (grant number RB13-0011) and the Swedish Cancer Society. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arroyo Mühr, L.S., Lagheden, C., Lei, J. et al. Deep sequencing detects human papillomavirus (HPV) in cervical cancers negative for HPV by PCR. Br J Cancer 123, 1790–1795 (2020). https://doi.org/10.1038/s41416-020-01111-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-01111-0
This article is cited by
-
Integrated genomic and transcriptomic analysis reveals the activation of PI3K signaling pathway in HPV-independent cervical cancers
British Journal of Cancer (2024)
-
Detection of circulating cell-free HPV DNA of 13 HPV types for patients with cervical cancer as potential biomarker to monitor therapy response and to detect relapse
British Journal of Cancer (2023)
-
A comprehensive HPV-STI NGS assay for detection of 29 HPV types and 14 non-HPV sexually transmitted infections
Infectious Agents and Cancer (2022)
-
Metatranscriptome analysis in human papillomavirus negative cervical cancers
Scientific Reports (2022)
-
Using HPV-meta for human papillomavirus RNA quality detection
Scientific Reports (2022)