Abstract
We aimed to examine whether statin users have a lower risk of hepatocellular carcinoma (HCC) after careful consideration of prevalent statin use and cholesterol levels. During a mean prospective follow-up of 8.4 years in 400,318 Koreans, 1686 individuals were diagnosed with HCC. When prevalent users were included, HCC risk was reduced by >50% in statin users, regardless of adjustment for total cholesterol (TC). When prevalent users were excluded, new users who initiated statins within 6 months after baseline had a 40% lower risk of HCC (hazard ratio [HR] = 0.59) in a TC-unadjusted analysis. However, this relationship disappeared (HR = 1.16, 95% CI = 0.80–1.69) after adjusting for TC levels measured within 6 months before statin initiation. TC levels had strong inverse associations with HCC in each model. High cholesterol levels at statin initiation, not statin use, were associated with reduced risk of HCC. Our study suggests no protective effect of statins against HCC.
Similar content being viewed by others
Background
Over 20 observational studies have suggested that statins protect against hepatocellular carcinoma (HCC) development.1,2,3,4,5 Most of those studies included individuals who had been using statins for some time before enrolment, although the inclusion of prevalent users can introduce biases in observational studies of efficacy.6 Hypercholesterolemia is the main indication for statin use. Blood cholesterol levels have been reported to have inverse associations with HCC in several studies.7 However, few studies of statin efficacy have controlled for cholesterol concentrations.8 Our prospective cohort study aimed to examine whether statin use was associated with a reduced risk of HCC after careful consideration of cholesterol levels and exclusion of prevalent users.
Methods
The main study cohort (n = 400,318), comprising participants in 2004–2007 health examinations administered through the National Health Insurance Service (NHIS) of Korea (Fig. S1), was followed-up until December 31, 2013 for HCC incidence via record linkage to hospital discharge records.9 Hazard ratios (HRs) for HCC incidence were calculated using Cox proportional hazards models after adjustment for multiple confounders (see Table 1 footnote). Information on statin use (atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin) was collected from the NHIS prescription database. Defined daily doses (DDDs) were used to quantify statin usage. Total cholesterol (TC), glucose, and alanine aminotransferase were assayed using fasting serum samples. Body mass index was measured. Smoking status, alcohol use, physical activity, and history of cancer were assessed via a questionnaire in previous (2002–2003) health examinations. More details on the study methods can be found in the supplemental text.
Results
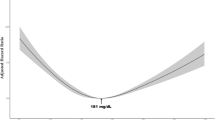
During 8.4 mean years of follow-up, 1686 individuals were diagnosed with HCC. They were more likely to be statin non-users than persons without HCC (Table S1). Statin users tended to be older, female, never-smokers, and high-income earners and to have higher TC levels, diabetes, and less liver disease than non-users (Table S2). In the analysis that included prevalent users (Table S3), the risk of HCC was generally reduced by >50% in statin users, regardless of dosage and adjustment for TC. When prevalent users were excluded, new users who initiated statins within 6 months after baseline had a 40% lower risk of HCC (HR = 0.59) in a TC-unadjusted analysis. However, this relationship disappeared (HR = 1.16, 95% CI = 0.80–1.69) after adjusting for baseline TC levels measured within 6 months before statin initiation (Table 1, Fig. 1). Additionally, when stratified analyses by TC levels were done, new users did not have a lowered risk of HCC in any TC group (Table S4)
The first 6 months of follow-up were excluded to minimise immortal time bias. HCC-free probability and 95% confidence intervals were calculated using Cox proportional hazard models after adjustment for age at baseline, sex, pre-existing diabetes, smoking status, alcohol use, physical activity, hepatitis B virus infection, hepatitis C virus infection, liver cirrhosis, body mass index, alanine aminotransferase levels, and total cholesterol levels (when applicable).
.
Interestingly, statin use within 2 years after baseline was associated with an ~30% lower risk of HCC than non-use in the multivariable-adjusted analysis (HR = 0.69, Table 1). In dose-risk analyses, the low- to moderate-dosage groups of ≤182 (HR = 0.71) and 183–365 (HR = 0.48) cDDDs within 2 years had lower risks, but not the highest dosage group of >365 cDDDs (HR = 1.09). The sensitivity analyses that excluded statin users within the past 2 years to minimise immortal time bias showed similar results (Table S5). When the timing of statin initiation was considered, statin initiation 1 year after baseline (HR = 0.47), but not within 6 months after baseline (HR = 1.12), was associated with lowered risk (Table 1). When only those who initiated statins within 1 year after baseline were considered as users, statin users (HR = 0.94) and each dosage group within 2 years after baseline had no lowered risk. Meanwhile, strong inverse associations with TC were found in each model.
Discussion
In the analysis that included prevalent users, statin users showed a >50% risk reduction for HCC. When prevalent users were excluded, new users also had a lower risk of HCC in a TC-unadjusted analysis. However, after adjustment for baseline TC levels measured within 6 months before statin initiation, the protective effects of statins against HCC disappeared. Blood TC levels had a strong inverse association with HCC incidence.
A major bias related to prevalent users is the inability to control for confounders that may be altered by statins.10 Confounding by indication is another source of bias. When associations between statin use and HCC risk are examined, the general indication for statins (i.e., cholesterol levels) is a confounder, since cholesterol levels have been inversely associated with HCC.7 Therefore, cholesterol levels are a key contributor both to prevalent user bias and to confounding by indication. Surprisingly, few studies on the effects of statin use on HCC have carefully accounted for cholesterol levels and excluded prevalent users.8 A few recent studies applied new-user designs, but, unfortunately, cholesterol levels were not adjusted for or most cholesterol measurements were made after statin use.3,5 However, our study demonstrated that adjustment for cholesterol levels cannot eliminate bias, especially when prevalent users or cholesterol levels measured after statin initiation were included in the analysis, and that measurements made 0.5–1 year before statin initiation may not reflect the values at the time of the decision to initiate statins. Meanwhile, it is possible that physicians avoid prescribing statins for individuals with liver disease. However, after adjustment for statin-unaffected TC, new users had no beneficial effect regardless of liver disease status (Table S6). A secondary analysis of randomised trials of statins on cardiovascular outcomes, which are free from these biases, showed that statins had no protective effects against HCC development (incidence rate ratio = 1.06), although this finding was based on a small number of cases (n = 68).11
Our finding that later initiation or recent use of statins was associated with lower risk are in accordance with the findings of a UK study that recent users, but not past users, had lower risks of HCC.4 Immortal time bias may partly, but not entirely, explain this result, since excluding the first 2 years of follow-up modestly changed the findings. The most recent statin users may have a lowered risk, probably not because of statin use, but because of their recent high cholesterol levels, which were not prominent at baseline; since lower cholesterol levels are a marker of liver disease and its severity,12 cholesterol levels (the indication for statins) have strong inverse associations with HCC.7 Analyses of cholesterol levels in the 2002–2003 and 2004–2007 health examinations showed that new initiators had higher levels than current non-users (intergroup difference; groups 5–8 vs. groups 1–4 in 2002–2003; groups 2, 6, 10, 14 vs groups 1, 5, 9, 13 in 2004–2007) and in recent measurements than in previous measurements (intragroup difference; group 2 in 2004–2007 vs. in 2002–2003), while those who discontinued statins had lower levels in recent measurements than in previous measurements (intragroup difference; group 5 in 2004–2007 vs. in 2002–2003) (Table S7).
Previous studies have reported non-linear associations of statin dosage with HCC risk: relatively small cumulative dosages had comparable or even greater preventive effects, compared with higher dosages.2,3,4,8 Our results related to the recency of statin use may explain these non-linear associations, as smaller-dosage users had lowered risk because they tended to include more recent initiators than their counterparts, although this group did also include early quitters.
As a prospective cohort study, biases related to the retrospective design, such as recall and selection biases, were minimised. Nearly complete follow-up for HCC via linkage to a national database is a further strength. Nonetheless, our study has limitations. Relatively few people received statins, and the cumulative dosages seemed to be relatively low in users. Our study participants were homogeneously Korean, which might affect the generalisability of the study. Additionally, our finding that statin use was associated with a lower risk of HCC when prevalent users were included or cholesterol levels were not adjusted for, as in previous observational studies, enhances the generalisability of our findings.
In conclusion, new statin users experienced no beneficial effects against HCC after adjustment for TC levels measured within 6 months before statin use, whereas TC levels had a strong inverse association with HCC. Observational studies evaluating the effects of statins against HCC should include only new users, after careful consideration of cholesterol levels and other confounders measured just before statin initiation to minimise biases. Overall, our study suggests no protective effect of statins against HCC.
References
Singh, S., Singh, P. P., Singh, A. G., Murad, M. H. & Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 144, 323–332 (2013).
Zhong, G. C., Liu, Y., Ye, Y. Y., Hao, F. B., Wang, K. & Gong, J. P. Meta-analysis of studies using statins as a reducer for primary liver cancer risk. Sci. Rep. 6, 26256 (2016).
Kim, G., Jang, S. Y., Nam, C. M. & Kang, E. S. Statin use and the risk of hepatocellular carcinoma in patients at high risk: a nationwide nested case-control study. J. Hepatol. 68, 476–484 (2018).
McGlynn, K. A., Hagberg, K., Chen, J., Graubard, B. I., London, W. T., Jick, S. et al. Statin use and risk of primary liver cancer in the clinical practice research datalink. J. Natl Cancer. Inst. 107, djv009 (2015).
Simon, T. G., Duberg, A. S., Aleman, S., Hagstrom, H., Nguyen, L. H., Khalili, H. et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide swedish population. Ann. Intern. Med. 171, 318–327 (2019).
Danaei, G., Tavakkoli, M. & Hernan, M. A. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am. J. Epidemiol. 175, 250–262 (2012).
Chiang, C. H., Lee, L. T., Hung, S. H., Lin, W. Y., Hung, H. F., Yang, W. S. et al. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology 59, 2207–2215 (2014).
Friedman, G. D., Achacoso, N., Fireman, B. & Habel, L. A. Statins and reduced risk of liver cancer: evidence for confounding. J. Natl Cancer Inst. 108, dwj109 (2016).
Yi, S. W., Choi, J. S., Yi, J. J., Lee, Y. H. & Han, K. J. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: a prospective cohort study in Korea. Cancer 124, 2748–2757 (2018).
Ray, W. A. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158, 915–920 (2003).
Cholesterol Treatment Trialists Collaboration, Emberson, J. R., Kearney, P. M., Blackwell, L., Newman, C., Reith, C. et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE 7, e29849 (2012).
Ghadir, M. R., Riahin, A. A., Havaspour, A., Nooranipour, M. & Habibinejad, A. A. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat. Mon. 10, 285–288 (2010).
Acknowledgements
The authors thank the staff of the Big Data Steering Department at the NHIS for providing the data and support.
Author information
Authors and Affiliations
Contributions
S.W.Y. conceived the study concept and design and acquired data. S.W.Y. performed statistical analysis and wrote the first draft. S.W.Y., S.H.K., K.J.H., J.J.Y., and H.O. analysed and interpreted data and contributed to critical revision of the manuscript. All authors have read and approved of the final submitted version of the manuscript. S.W.Y. is the study guarantor.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of Catholic Kwandong University (CKU-16-01-0301). Informed consent was waived because the anonymised data was used that was provided by NHIS according to strong confidentiality protocol.
Consent to publish
Not applicable.
Data availability
The data underlying the results presented in the study are available from the National Health Insurance Service (NHIS) (http://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do). Applicants to use the data should contact the NHIS (Office of big data operation, +82-33-736-2469) for further information.
Competing interests
The authors declare no competing interests.
Funding information
There was no funding specific for the current work.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yi, SW., Kim, S.H., Han, K.J. et al. Higher cholesterol levels, not statin use, are associated with a lower risk of hepatocellular carcinoma. Br J Cancer 122, 630–633 (2020). https://doi.org/10.1038/s41416-019-0691-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-019-0691-3
This article is cited by
-
Low-density lipoprotein cholesterol and risk of hepatocellular carcinoma: a Mendelian randomization and mediation analysis
Lipids in Health and Disease (2023)
-
A positive feedback between cholesterol synthesis and the pentose phosphate pathway rather than glycolysis promotes hepatocellular carcinoma
Oncogene (2023)
-
Impact of overweight and obesity on the risk of hepatocellular carcinoma: a prospective cohort study in 14.3 million Koreans
British Journal of Cancer (2022)
-
Cholesterol and hepatocellular carcinoma risk: reliable and actionable?
British Journal of Cancer (2021)