Abstract
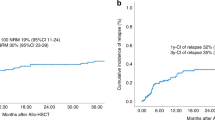
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a currative treatment modality for diffuse large B-cell lymphoma (DLBCL) because of the intrinsic graft-versus-lymphoma effect. However, limited information is available regarding which patients with relapsed or refractory DLBCL are likely to benefit from allo-HSCT. We retrospectively analyzed data from 1268 DLBCL patients who received allo-HSCT. The overall survival and progression-free survival (PFS) rates were 30.3% and 21.6% at 3 years, respectively. Multivariate analysis revealed that stable or progressive disease at transplantation, male patient, poorer performance status at transplantation, and shorter intervals from previous transplantation were associated independently with a lower PFS. Four prognostic factors were used to construct a prognostic index for PFS, predicting 3-year PFS of 55.4%, 43.7%, 20.4% and 6.6%, respectively. The prognostic model predicted relapse rates following allo-HSCT accordingly (P < 0.0001), whereas did not predict transplantation-related mortality (P = 0.249). The prognostic index can identify a subgroup of DLBCL patients who benefit from allo-HSCT and it is worthwhile to evaluate whether this model is also applicable to patients undergoing allo-HSCT in cases of relapse after chimeric antigen receptor engineered T-cell therapy, although the application of allo-HSCT has been declining with the increase of novel immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the Japan Society for Hematopoietic Cell Transplantation (JSHCT) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of JSHCT.
References
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242.
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5.
Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR Tcell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Abramson, Palomba JS, Gordon LI ML, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. All ZUMA-7 investigators and contributing kite members. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54.
Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386:629–39.
Abramson JS, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. 2023;141:1675–84.
Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8.
Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2020;38:155–65.
Assi R, Masri N, Dalle IA, El-Cheikh J, Ghanem H, Bazarbachi A. Polatuzumab vedotin: current role and future applications in the treatment of patients with diffuse large B-cell lymphoma. Clin Hematol Int. 2021;3:21–26.
Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J Clin Oncol. 2023;41:2238–47.
Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2022;387:2220–31.
Bishop MR, Dean RM, Steinberg SM, Odom J, Pavletic SZ, Chow C, et al. Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann Oncol. 2008;19:1935–40.
Sirvent A, Dhedin N, Michallet M, Mounier N, Faucher C, Yakoub-Agha I, et al. Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Biol Blood Marrow Transplant. 2010;16:78–85.
van Kampen RJ, Canals C, Schouten HC, Nagler A, Thomson KJ, Vernant JP, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. 2011;29:1342–8.
Hamadani M, Saber W, Ahn KW, Carreras J, Cairo MS, Fenske TS, et al. Impact of pretransplantation conditioning regimens on outcomes of allogeneic transplantation for chemotherapy-unresponsive diffuse large B cell lymphoma and grade III follicular lymphoma. Biol Blood Marrow Transpl. 2013;19:746–53.
Glass B, Hasenkamp J, Wulf G, Dreger P, Pfreundschuh M, Gramatzki M, et al. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol. 2014;15:757–66.
Klyuchnikov E, Bacher U, Kroll T, Shea TC, Lazarus HM, Bredeson C, et al. Allogeneic hematopoietic cell transplantation for diffuse large B cell lymphoma: who, when and how? Bone Marrow Transplant. 2014;49:1–7.
Fenske TS, Ahn KW, Graff TM, DiGilio A, Bashir Q, Kamble RT, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016;174:235–48.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Reagan PM, Miklos DB, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021;5:4149–55.
Hamadani M, Gopal AK, Pasquini M, Kim S, Qiu X, Ahmed S, et al. Allogeneic transplant and CAR-T therapy after autologous transplant failure in DLBCL: a noncomparative cohort analysis. Blood Adv. 2022;6:486–94.
Mussetti A, Bento L, Bastos-Oreiro M, Rius-Sansalvador B, Albo C, Bailen R, et al. Role of allogeneic hematopoietic cell transplant for relapsed/refractory aggressive B-cell lymphomas in the CART era. Bone Marrow Transpl. 2023;58:673–9.
Zurko J, Ramdial J, Shadman M, Ahmed S, Szabo A, Iovino L, et al. Allogeneic transplant following CAR T-cell therapy for large B-cell lymphoma. Haematologica. 2023;108:98–109.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–7.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transpl. 2009;15:367–9.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. International harmonization project on lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Epperla N, Ahn KW, Khanal M, Litovich C, Ahmed S, Ghosh N, et al. Impact of Reduced-Intensity Conditioning Regimens on Outcomes in Diffuse Large B Cell Lymphoma Undergoing Allogeneic Transplantation. Transpl Cell Ther. 2021;27:58–66.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Scott DW, King RL, Staiger AM, Ben-Neriah S, Jiang A, Horn H, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131:2060–4.
Karube K, Enjuanes A, Dlouhy I, Jares P, Martin-Garcia D, Nadeu F, et al. Integrating genomic alterations in diffuse large B-cell lymphoma identifies new relevant pathways and potential therapeutic targets. Leukemia. 2018;32:675–84.
Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24:679–90.
Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396–407.
Wright GW, Huang DW, Phelan JD, Coulibaly ZA, Roulland S, Young RM, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer cell. 2020;37:551–68.
Sauter CS, Matasar MJ, Meikle J, Schoder H, Ulaner GA, Migliacci JC, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015;125:2579–81.
Shah NN, Ahn KW, Litovich C, He Y, Sauter C, Fenske TS, et al. Is autologous transplant in relapsed DLBCL patients achieving only a PET+ PR appropriate in the CAR T-cell era? Blood. 2021;137:1416–23.
Acknowledgements
We thank the physicians and data managers at the institutes who contributed valuable data regarding adult lymphoma-related transplantation to the JSHCT. We also thank all members of the data management committees of the JSHCT.
Author information
Authors and Affiliations
Contributions
KK, TS, TI, KY, KM, JS, and RS designed the study; KK, GY, SK, KI, YU, YM, JI, NH, TE, HN, HK, KS, OM, TF, and YA collected the data; KK and TS performed the statistical analysis. KK and TS wrote the manuscript and created the figures and tables; all authors critically reviewed the manuscript and read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
KK; Honoraria: Bristol-Myers Squibb, Celgene, Dainippon-Sumitomo, Janssen, Kyowa Kirin, MSD, Ono; Consulting or Advisory Role: AbbVie, AstraZeneca, Celgene, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Janssen, Novartis; Research Funding: AbbVie, MSD, Bristol-Myers Squibb, Celgene, Chugai, Daiichi Sankyo, Eisai, Janssen, Kyowa Kirin, Novartis, Ono. KM; Honoraria: Chugai Pharma, SymBio Pharmaceuticals, Janssen, Eisai, Nippon Shinyaku, AstraZeneca, Bristol-Myers Squibb, Meiji Seika Pharma, Abbvie, Novartis, Incyte, Asahi Kasei; Research Funding: Eisai, Takeda, Nipoon Shinyaku, Otsuka, Chugai Pharma, Asahi Kasei, Sumitomo Dainippon Pharma Oncology, Zenyaku Kogyo. KS; Honoraria: Janssen, Sanofi, Takeda. Ritsuro Suzuki; Honoraria: Chugai, Kyowa Kirin, Takeda, Meiji Seika, Abbvie, AstraZeneca, Janssen, Otsuka, Eisai, Nippon Shinyaku, Elli Lilly, Amgen, Novartis, Nihon Kayaku, Sumitomo; Research Funding: Chugai, Kyowa Kirin, Takeda, Eisai, Shionogi, Otsuka, Taiho.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kato, K., Sugio, T., Ikeda, T. et al. Outcomes of allogeneic hematopoietic stem cell transplantation for relapsed or refractory diffuse large B-cell lymphoma. Bone Marrow Transplant 59, 306–314 (2024). https://doi.org/10.1038/s41409-023-02156-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02156-4