Abstract
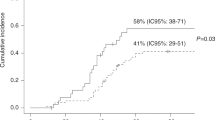
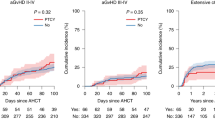
Post-transplant cyclophosphamide (PTCy) is increasingly used to reduce graft-versus-host disease after hematopoietic cell transplantation (HCT); however, it might be associated with more infections. All patients who were ≥2 years old, receiving haploidentical or matched sibling donor (Sib) HCT for acute leukemias or myelodysplastic syndrome, and either calcineurin inhibitor (CNI)- or PTCy-based GVHD prophylaxis [Haploidentical HCT with PTCy (HaploCy), 757; Sibling with PTCy (SibCy), 403; Sibling with CNI-based (SibCNI), 1605] were included. Most bacterial infections occurred within the first 100 days; 953 patients (34.5%) had at least 1 infection and 352 patients (13%) had ≥2 infections. Patients receiving PTCy had a greater incidence of bacterial infections by day 180 [HaploCy 46%; SibCy 48%; SibCNI 35%; p < 0.001]. Compared with the SibCNI without infection cohort, 1.99-fold, 3.33-fold, 2.78-fold, and 2.53-fold increased TRM was seen for the HaploCy cohort without infection and HaploCy, SibCy, and SibCNI cohorts with infection, respectively. Bacterial infections increased mortality [HaploCy (HR1.84, 99% CI: 1.45–2.33, p < 0.0001), SibCy cohort (HR,1.68, 99% CI: 1.30–2.19, p < 0.0001), and SibCNI cohort (HR,1.76, 99% CI: 1.43–2.16, p < 0.0001). PTCy was associated with increased bacterial infections regardless of donor, and bacterial infections were associated with increased mortality irrespective of GVHD prophylaxis. Patients receiving PTCy should be monitored carefully for bacterial infections following PTCy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Materials described in the manuscript, including all relevant raw data, belongs to CIBMTR and will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality per discussion with CIBMTR.
References
Vydra J, Shanley RM, George I, Ustun C, Smith AR, Weisdorf DJ, et al. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:764–70.
Ballen K, Woo Ahn K, Chen M, Abdel-Azim H, Ahmed I, Aljurf M, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2016;22:1636–45.
Papanicolaou GA, Ustun C, Young JH, Chen M, Kim S, Ahn KW, et al. Bloodstream infection (BSI) due to Vancomycin-Resistant Enterococcus (VRE) is associated with increased mortality after hematopoietic cell transplantation for acute leukemia and myelodysplastic syndrome: a multicenter, retrospective cohort study. Clin Infect Dis. 2019;69:1771–9.
Ustun C, Kim S, Chen M, Beitinjaneh AM, Brown VI, Dahi PB, et al. Increased overall and bacterial infections following myeloablative allogeneic HCT for patients with AML in CR1. Blood Adv. 2019;3:2525–36.
Ustun C, Young JH, Papanicolaou GA, Kim S, Ahn KW, Chen M, et al. Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2019;54:1254–65.
Akhmedov M, Klyasova G, Kuzmina L, Fedorova A, Vasilyeva V, Drokov M, et al. Incidence, etiology, risk factors, and outcomes of pre-engraftment bloodstream infections after first and second allogeneic hematopoietic cell transplantation. Transpl Infect Dis. 2022;24:e13842.
Dandoy CE, Kim S, Chen M, Ahn KW, Ardura MI, Brown V, et al. Incidence, risk factors, and outcomes of patients who develop mucosal barrier injury-laboratory confirmed bloodstream infections in the first 100 days after allogeneic hematopoietic stem cell transplant. JAMA Netw Open. 2020;3:e1918668.
Takagi S, Ogura S, Araoka H, Uchida N, Mitsuki T, Yuasa M, et al. The impact of graft cell source on bloodstream infection in the first 100 days after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2021;56:1625–34.
Dandoy CE, Ardura MI, Papanicolaou GA, Auletta JJ. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: new considerations for a persistent nemesis. Bone Marrow Transpl. 2017;52:1091–106.
Nathan S, Ustun C. Complications of stem cell transplantation that affect infections in stem cell transplant recipients, with analogies to patients with hematologic malignancies. Infect Dis Clin North Am. 2019;33:331–59.
Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–8.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50.
Saliba RM, Alousi AM, Pidala J, Arora M, Spellman SR, Hemmer MT, et al. Characteristics of Graft-Versus-Host Disease (GvHD) after post-transplantation cyclophosphamide versus conventional GvHD prophylaxis. Transpl Cell Ther. 2022;28:681–93.
McCurdy SR, Luznik L. How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide. Hematol Am Soc Hematol Educ Program. 2019;2019:513–21.
Webster JA, Luznik L, Tsai HL, Imus PH, DeZern AE, Pratz KW, et al. Allogeneic transplantation for Ph+ acute lymphoblastic leukemia with posttransplantation cyclophosphamide. Blood Adv. 2020;4:5078–88.
Goldsmith SR, Slade M, DiPersio JF, Westervelt P, Lawrence SJ, Uy GL, et al. Cytomegalovirus viremia, disease, and impact on relapse in T-cell replete peripheral blood haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide. Haematologica. 2016;101:e465–e468.
Huntley D, Gimenez E, Pascual MJ, Hernandez-Boluda JC, Gago B, Vazquez L, et al. Incidence, features, and outcomes of cytomegalovirus DNAemia in unmanipulated haploidentical allogeneic hematopoietic stem cell transplantation with post-transplantation cyclophosphamide. Transpl Infect Dis. 2020;22:e13206.
Jamy O, Hebert C, Dunn-Valadez S, Magnusson T, Watts N, McGwin G, et al. Risk of cytomegalovirus infection with post-transplantation cyclophosphamide in haploidentical and hla-matched unrelated donor transplantation. Transplant Cell Ther. 2022;28:213.e1-213.e6.
Goldsmith SR, Abid MB, Auletta JJ, Bashey A, Beitinjaneh A, Castillo P, et al. Posttransplant cyclophosphamide is associated with increased cytomegalovirus infection: a CIBMTR analysis. Blood. 2021;137:3291–305.
Singh A, Dandoy CE, Chen M, Kim S, Mulroney CM, Kharfan-Dabaja MA, et al. Post-transplantation cyclophosphamide is associated with an increase in non-cytomegalovirus herpesvirus infections in patients with acute leukemia and myelodysplastic syndrome. Transp Cell Ther. 2022;28:48. e41–48.e10.
Mulroney CM, Abid MB, Bashey A, Chemaly RF, Ciurea SO, Chen M, et al. Incidence and impact of community respiratory viral infections in post‐transplant cyclophosphamide‐based graft‐versus‐host disease prophylaxis and haploidentical stem cell transplantation. Br J Haematol. 2021;194:145–57.
Esquirol A, Pascual MJ, Kwon M, Pérez A, Parody R, Ferra C, et al. Severe infections and infection-related mortality in a large series of haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. Bone Marrow Transpl. 2021;56:2432–44.
Mikulska M, Raiola AM, Galaverna F, Balletto E, Borghesi ML, Varaldo R, et al. Pre-engraftment bloodstream infections after allogeneic hematopoietic cell transplantation: impact of T cell-replete transplantation from a haploidentical donor. Biol Blood Marrow Transpl. 2018;24:109–18.
Oltolini C, Greco R, Galli L, Clerici D, Lorentino F, Xue E, et al. Infections after allogenic transplant with post-transplant cyclophosphamide: impact of donor HLA matching. Biol Blood Marrow Transpl. 2020;26:1179–88.
manual Cfi. Q428- 440: infection. 2020; Available from: https://www.cibmtr.org/manuals/fim/1/en/topic/f2100-q428-440 Accesed 5 Mar 2022.
Tomblyn M, Young JA, Haagenson MD, Klein JP, Trachtenberg EA, Storek J, et al. Decreased infections in recipients of unrelated donor hematopoietic cell transplantation from donors with an activating KIR genotype. Biol Blood Marrow Transpl. 2010;16:1155–61.
See I, Iwamoto M, Allen-Bridson K, Horan T, Magill SS, Thompson ND. Mucosal barrier injury laboratory-confirmed bloodstream infection: results from a field test of a new National Healthcare Safety Network definition. Infect Control Hosp Epidemiol. 2013;34:769–76.
Epstein L, See I, Edwards JR, Magill SS, Thompson ND. Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infections (MBI-LCBI): descriptive analysis of data reported to National Healthcare Safety Network (NHSN), 2013. Infect Control Hosp Epidemiol. 2016;37:2–7.
Kim S, Logan B, Riches M, Chen M, Ahn KW. Statistical methods for time-dependent variables in hematopoietic cell transplantation studies. Transpl Cell Ther. 2021;27:125–32.
Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1:145–56.
Crocchiolo R, Bramanti S, Vai A, Sarina B, Mineri R, Casari E, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis. 2015;17:242–9.
Fayard A, Daguenet E, Blaise D, Chevallier P, Labussiere H, Berceanu A, et al. Evaluation of infectious complications after haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide following reduced-intensity and myeloablative conditioning: a study on behalf of the Francophone Society of Stem Cell Transplantation and Cellular Therapy (SFGM-TC). Bone Marrow Transpl. 2019;54:1586–94.
Slade M, Goldsmith S, Romee R, DiPersio JF, Dubberke ER, Westervelt P, et al. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl Infect Dis. 2017;19:e12629.
Irene GC, Albert E, Anna BV, Rahinatu A, Silvana N, Silvana S, et al. Patterns of infection and infectious-related mortality in patients receiving post-transplant high dose cyclophosphamide as graft-versus-host-disease prophylaxis: impact of HLA donor matching. Bone Marrow Transpl. 2021;56:818–27.
Nagler A, Labopin M, Houhou M, Aljurf M, Mousavi A, Hamladji RM, et al. Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a study from the acute leukemia working party of the european society for blood and marrow transplantation. J Hematol Oncol. 2021;14:53.
Khimani F, Ranspach P, Elmariah H, Kim J, Whiting J, Nishihori T, et al. Increased infections and delayed CD4(+) T Cell but Faster B Cell immune reconstitution after post-transplantation cyclophosphamide compared to conventional GVHD prophylaxis in allogeneic transplantation. Transpl Cell Ther. 2021;27:940–8.
Salas MQ, Charry P, Puerta-Alcalde P, Martinez-Cibrian N, Solano MT, Serrahima A, et al. Bacterial bloodstream infections in patients undergoing allogeneic hematopoietic cell transplantation with post-transplantation cyclophosphamide. Transplant Cell Ther. 2022;28:850.e1-850.e10.
Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2012;18:1835–44.
Holtan SG, Hamadani M, WU J, AL Malki MM, Runaas L, Elmariah H, et al. Post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil as the new standard for Graft-Versus-Host Disease (GVHD) prophylaxis in reduced intensity conditioning: results from phase III BMT CTN 1703. Blood. 2022;140:LBA-4–LBA-4.
Acknowledgements
Data Use Statement: CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Funding
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); 75R60222C00011 from the Health Resources and Services Administration (HRSA); N00014–21–1–2954 and N00014-23-1-2057 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Angiocrine; Anthem; Astellas Pharma US; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medexus Pharma; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pluristem; PPD Development, LP; Regimmune; Sanofi; Sanofi-Aventis U.S. Inc.; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.
Author information
Authors and Affiliations
Contributions
CU and GAP conceived the concept, analyzed data, searched literature, wrote and edited the article; MR, MC and SK created and analyzed data, did statistical analyses, wrote and edited the article; JJA, MVB, MB, JC, LG, JAH, HL, PNM, SN, MDS, JRW, RFC, CED, MAP analyzed data, searched literature, wrote and edited the article.
Corresponding author
Ethics declarations
Competing interests
MVB reports compensation (honoraria) from from United Medical, Astra Zeneca, and Merck Sharp Dohme; and research support from National Health Institute- National Cancer Institute (NIH-NCI), National Council for Scientific and Technological Development (CNPq) and FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo. MB reports research support (institution) from Novartis. JC reports consulting fees (Advisory Board Membership) for Jazz Pharmaceuticals, Pfizer, and Amgen; Data Safety Monitoring Board member for Allovir Inc.; and stocks with Actinium Pharmaceuticals, Bluebird Bio/2Seventy, Dynavax Technologies, aTyr Pharma, Gamida Cell, Miragen Therapeutics, Mustang Bio, Novavax, Ovid Therapeutics, Sorrento Therapeutics, TG Therapeutics, Vaxart, and Veru. JAH reports compensation (consulting) for Karius; research support from Karius; and significant payments (Scientific Advisory Board) for Karius (limit $10,000). HL reports compensation (advisor/consultant/speaker) for Agios, Pfizer, CTI Biopharm, Nkarta, Servier; research support (funding paid to my institution) for clinical trials from Miltenyi Biotec, Alexion, Astellas, Nkarta, ImmuOnco, Cellularity and Marker. PNM reports Speaker’s Bureau participation for Incyte and Kite; and consultancy for Incyte. JRW reports compensation (consultant) for Cidara, Celgene, Merck, Shire, Orca and Ansun. RFC reports compensation (advisor/consultant/speaker) for Merck, Takeda, Karius, Therapeutics Partners, Ansun, Eurofins-Viracor, Genentech, Qiagen, Oxford Immunotec, Astellas, Roche, Aseptico, AiCuris, and Shinogi; and significant payments (grants and funding paid to my institution for research) from Merck, Ansun, AiCuris, Eurofins-Viracor, Karius, Genentech, Freestyle, and Takeda. MAP reports compensation (honoraria) from Adicet, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma; Data Safety Monitoring Board participation for Cidara Therapeutics, Medigene, and Sellas Life Sciences; scientific advisory board participation for NexImmune; ownership interests in NexImmune and Omeros; significant payments (honoraria in excess of $5000) from Bristol-Myers Squibb, Incyte, Kite/Gilead, Merck, Nektar Therapeutics, Novartis, Omeros, Cidara Therapeutics (DSMB) and research support (institutional in excess of $5000) for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis; and equity interest in Omeros. MR is an employee of IQVIA Biotech, a clinical research organization. GAP reports compensation (consultant/other) for Allovir, Astellas, Cidara, Merck, Takeda, Therapeutics Partners and Octapharma; and research funding (paid to my institution) from Merck and Takeda.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ustun, C., Chen, M., Kim, S. et al. Post-transplantation cyclophosphamide is associated with increased bacterial infections. Bone Marrow Transplant 59, 76–84 (2024). https://doi.org/10.1038/s41409-023-02131-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02131-z