Abstract
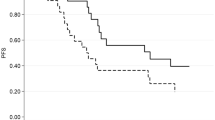
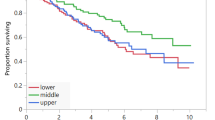
Recently there has been a growing interest in evaluating body composition as a marker for prognosis in cancer patients. The association of body composition parameters and outcomes has not been deeply investigated in patients with autologous hematopoietic stem cell transplantation (HSCT) recipients with non-Hodgkin lymphoma (NHL). We conducted a retrospective cohort study of 264 NHL patients who received autologous HSCT. PreHSCT abdominal CT scans at the levels of L3 were assessed for body composition measures. We evaluated sarcopenia, myosteatosis, high visceral adipose tissue (VAT) and high visceral adipose tissue density (VATD). Using multivariable Cox proportional regression, we analyzed the association of clinical and transplant-related characteristics with overall survival (OS), relapse-free survival (RFS), and non-relapse mortality (NRM). In a multivariate regression model, patients with higher VATD had worse OS (HR 1.78; 95% confidence intervals CI 1.08–2.95, p = 0.02) and worse NRM (HR 2.31 95% CI 1.08–4.95, p = 0.02) than with lower VATD. Patients with lower levels of VAT also had worse RFS (HR 1.49 95% CI 1.03–2.15, p = 0.03). Sarcopenia and myosteatosis were not associated with outcomes. High pre-transplant VATD was associated with lower OS and higher NRM, and low pre-transplant VAT was associated with worse RFS in patients with NHL undergoing autologous HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to confidentiality reasons but are available from the corresponding author on reasonable request.
References
WCRF/AICR. Diet, nutrition, physical activity and cancer: a global perspective. A summary of the third Expert Report. Report no.: Expert Report 2018. World Cancer Research Fund/American Institute for Cancer Research; 2018.
Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today. 2015;50:11728. https://doi.org/10.1097/NT.0000000000000092.
Cespedes Feliciano EM, Popuri K, Cobzas D, Baracos VE, Beg MF, Khan AD, et al. Evaluation of automated computed tomography segmentation to assess body composition and mortality associations in cancer patients. J Cachexia Sarcopenia Muscle. 2020. https://doi.org/10.1002/jcsm.12573.
Cespedes Feliciano EM, Chen WY, Bradshaw PT, Prado CM, Alexeeff S, Albers KB, et al. Adipose tissue distribution and cardiovascular disease risk among breast cancer survivors. J Clin Oncol. 2019:JCO1900286. https://doi.org/10.1200/JCO.19.00286.
Cespedes Feliciano EM, Chen WY, Lee V, Albers KB, Prado CM, Alexeeff S, et al. Body composition, adherence to anthracycline and taxane-based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol. 2019. https://doi.org/10.1001/jamaoncol.2019.4668.
Kroenke CH, Prado CM, Meyerhardt JA, Weltzien EK, Xiao J, Cespedes Feliciano EM, et al. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer. 2018;124:3008–15. https://doi.org/10.1002/cncr.31405.
Rollins KE, Gopinath A, Awwad A, Macdonald IA, Lobo DN. Computed tomography-based psoas skeletal muscle area and radiodensity are poor sentinels for whole L3 skeletal muscle values. Clin Nutr. 2019. https://doi.org/10.1016/j.clnu.2019.10.003.
Alipour O, Lee V, Tejura TK, Wilson ML, Memel Z, Cho J, et al. The assessment of sarcopenia using psoas muscle thickness per height is not predictive of post-operative complications in IBD. Scand J Gastroenterol. 2021;56:1175–81. https://doi.org/10.1080/00365521.2021.1958368.
Lee B, Bae YJ, Jeong WJ, Kim H, Choi BS, Kim JH. Temporalis muscle thickness as an indicator of sarcopenia predicts progression-free survival in head and neck squamous cell carcinoma. Sci Rep. 2021;11:19717. https://doi.org/10.1038/s41598-021-99201-3.
Go SI, Park MJ, Song HN, Kim HG, Kang MH, Kang JH, et al. A comparison of pectoralis versus lumbar skeletal muscle indices for defining sarcopenia in diffuse large B-cell lymphoma—two are better than one. Oncotarget. 2017;8:47007–19. https://doi.org/10.18632/oncotarget.16552.
Arayne AA, Gartrell R, Qiao J, Baird PN, Yeung JM. Comparison of CT derived body composition at the thoracic T4 and T12 with lumbar L3 vertebral levels and their utility in patients with rectal cancer. BMC Cancer. 2023;23:56. https://doi.org/10.1186/s12885-023-10522-0.
Vangelov B, Bauer J, Kotevski D, Smee RI. The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: a systematic review. Br J Nutr. 2022;127:722–35. https://doi.org/10.1017/S0007114521001446.
Sumransub N, Cao Q, Juckett M, Betts B, Holtan S, Jurdi NE, et al. Sarcopenia predicts inferior progression free survival in lymphoma patients treated with autologous hematopoietic stem cell transplantation. Transplant Cell Ther. 2023. https://doi.org/10.1016/j.jtct.2023.01.015.
Kapoor ND, Twining PK, Groot OQ, Pielkenrood BJ, Bongers MER, Newman ET, et al. Adipose tissue density on CT as a prognostic factor in patients with cancer: a systematic review. Acta Oncol. 2020;59:1488–95. https://doi.org/10.1080/0284186X.2020.1800087.
Aleixo GFP, Sheu M, Mirzai S, Majhail NS. Prognostic impact of adiposity in hematological malignancies: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2022. https://doi.org/10.1016/j.clml.2022.05.008.
Xiao J, Mazurak VC, Olobatuyi TA, Caan BJ, Prado CM. Visceral adiposity and cancer survival: a review of imaging studies. Eur J Cancer Care. 2018;27:e12611. https://doi.org/10.1111/ecc.12611.
Monirujjaman MD, Martin L, Stretch C, Mazurak VC. Adipose tissue radiodensity in chronic diseases: a literature review of applied methodologies. Immunometabolism. 2021;3:e210033.
da Cunha ADJ, Silveira MN, Takahashi MES, de Souza EM, Mosci C, Ramos CD, et al. Adipose tissue radiodensity: a new prognostic biomarker in people with multiple myeloma. Nutrition. 2021;86:111141. https://doi.org/10.1016/j.nut.2021.111141.
Shah NN, Ahn KW, Litovich C, Sureda A, Kharfan-Dabaja MA, Awan FT, et al. Allogeneic transplantation in elderly patients >/=65 years with non-Hodgkin lymphoma: a time-trend analysis. Blood Cancer J. 2019;9:97. https://doi.org/10.1038/s41408-019-0261-1.
Hegde A, Murthy HS. Frailty: the missing piece of the pre-hematopoietic cell transplantation assessment? Bone Marrow Transplant. 2018;53:3–10. https://doi.org/10.1038/bmt.2017.192.
Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer. 2018;26:861–8. https://doi.org/10.1007/s00520-017-3902-6.
Jabbour J, Manana B, Zahreddine A, Saade C, Charafeddine M, Bazarbachi A, et al. Sarcopenic obesity derived from PET/CT predicts mortality in lymphoma patients undergoing hematopoietic stem cell transplantation. Curr Res Transl Med. 2019;67:93–9. https://doi.org/10.1016/j.retram.2018.12.001.
Lin RJ, Michaud L, Lobaugh SM, Nakajima R, Mauguen A, Elko TA, et al. The geriatric syndrome of sarcopenia impacts allogeneic hematopoietic cell transplantation outcomes in older lymphoma patients. Leuk Lymphoma. 2020;61:1833–41. https://doi.org/10.1080/10428194.2020.1742909.
Camus V, Lanic H, Kraut J, Modzelewski R, Clatot F, Picquenot JM, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur J Haematol. 2014;93:9–18. https://doi.org/10.1111/ejh.12285.
Nakamura N, Ninomiya S, Matsumoto T, Nakamura H, Kitagawa J, Shiraki M, et al. Prognostic impact of skeletal muscle assessed by computed tomography in patients with acute myeloid leukemia. Ann Hematol. 2019;98:351–9. https://doi.org/10.1007/s00277-018-3508-1.
Jung J, Lee E, Shim H, Park JH, Eom HS, Lee H. Prediction of clinical outcomes through assessment of sarcopenia and adipopenia using computed tomography in adult patients with acute myeloid leukemia. Int J Hematol. 2021;114:44–52. https://doi.org/10.1007/s12185-021-03122-w.
Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71–87. https://doi.org/10.2165/11318100-000000000-00000.
Baker SD, van Schaik RH, Rivory LP, Ten Tije AJ, Dinh K, Graveland WJ, et al. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res. 2004;10:8341–50. https://doi.org/10.1158/1078-0432.CCR04-1371.
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–303. https://doi.org/10.4049/jimmunol.1003613.
GroDelta JP, Nattenmuller J, Hemmer S, Tichy D, Krzykalla J, Goldschmidt H, et al. Body fat composition as predictive factor for treatment response in patients with newly diagnosed multiple myeloma—subgroup analysis of the prospective GMMG MM5 trial. Oncotarget. 2017;8:68460–71. https://doi.org/10.18632/oncotarget.19536.
Shin DY, Kim A, Byun BH, Moon H, Kim S, Ko YJ, et al. Visceral adipose tissue is prognostic for survival of diffuse large B cell lymphoma treated with frontline R-CHOP. Ann Hematol. 2016;95:409–16. https://doi.org/10.1007/s00277-015-2571-0.
Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR. Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;145:102839. https://doi.org/10.1016/j.critrevonc.2019.102839.
Cypess AM. Reassessing human adipose tissue. N Engl J Med. 2022;386:768–79. https://doi.org/10.1056/NEJMra2032804.
Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–39. https://doi.org/10.1079/pns200194.
Rosenquist KJ, Massaro JM, Pedley A, Long MT, Kreger BE, Vasan RS, et al. Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. J Clin Endocrinol Metab. 2015;100:227–34. https://doi.org/10.1210/jc.2013-4296.
Deshmukh AS, Peijs L, Beaudry JL, Jespersen NZ, Nielsen CH, Ma T, et al. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab. 2019;30:963–75.e7. https://doi.org/10.1016/j.cmet.2019.10.001.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. https://doi.org/10.1172/JCI19246.
Ingram JR, Dougan M, Rashidian M, Knoll M, Keliher EJ, Garrett S, et al. PD-L1 is an activation independent marker of brown adipocytes. Nat Commun. 2017;8:647. https://doi.org/10.1038/s41467-017-00799-8.
Charette N, Vandeputte C, Ameye L, Bogaert CV, Krygier J, Guiot T, et al. Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: a post hoc analysis of two non-randomized phase II trials. BMC Cancer. 2019;19:134. https://doi.org/10.1186/s12885-019-5319-8.
Lee JW, Son MW, Chung IK, Cho YS, Lee MS, Lee SM. Significance of CT attenuation and F-18 fluorodeoxyglucose uptake of visceral adipose tissue for predicting survival in gastric cancer patients after curative surgical resection. Gastric Cancer. 2020;23:273–84. https://doi.org/10.1007/s10120-019-01001-2.
Lee JW, Lee SM, Chung YA. Prognostic value of CT attenuation and FDG uptake of adipose tissue in patients with pancreatic adenocarcinoma. Clin Radiol. 2018;73:1056. https://doi.org/10.1016/j.crad.2018.07.094.
Ebadi M, Moctezuma-Velazquez C, Meza-Junco J, Baracos VE, DunichandHoedl AR, Ghosh S, et al. Visceral adipose tissue radiodensity is linked to prognosis in hepatocellular carcinoma patients treated with selective internal radiation therapy. Cancers. 2020;12. https://doi.org/10.3390/cancers12020356.
Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–41. https://doi.org/10.1016/j.bbalip.2013.02.010.
Ahmadi N, Hajsadeghi F, Conneely M, Mingos M, Arora R, Budoff M, et al. Accurate detection of metabolically active “brown” and “white” adipose tissues with computed tomography. Acad Radiol. 2013;20:1443–7. https://doi.org/10.1016/j.acra.2013.08.012.
Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–47. https://doi.org/10.1016/j.cmet.2014.06.011.
Gullett NP, Mazurak VC, Hebbar G, Ziegler TR. Nutritional interventions for cancer-induced cachexia. Curr Probl Cancer. 2011;35:58–90. https://doi.org/10.1016/j.currproblcancer.2011.01.001.
Kuhn KS, Muscaritoli M, Wischmeyer P, Stehle P. Glutamine as indispensable nutrient in oncology: experimental and clinical evidence. Eur J Nutr. 2010;49:197–210. https://doi.org/10.1007/s00394-009-0082-2.
Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44:1124–32. https://doi.org/10.1016/j.ejca.2008.02.033.
Williams GR, Dunne RF, Giri S, Shachar SS, Caan BJ. Sarcopenia in the older adult with cancer. J Clin Oncol. 2021;39:2068–78. https://doi.org/10.1200/JCO.21.00102.
Hardee JP, Lynch GS. Current pharmacotherapies for sarcopenia. Expert Opin Pharmacother. 2019;20:1645–57. https://doi.org/10.1080/14656566.2019.1622093.
Detopoulou P, Tsiouda T, Pilikidou M, Palyvou F, Tsekitsidi E, Mantzorou M, et al. Changes in body weight, body composition, phase angle, and resting metabolic rate in male patients with stage IV non-small cell lung cancer undergoing therapy. Medicina (Kaunas). 2022;58. https://doi.org/10.3390/medicina58121779.
Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. https://doi.org/10.1210/jcem.87.2.8299.
Miyata H, Sugimura K, Motoori M, Fujiwara Y, Omori T, Yanagimoto Y, et al. Clinical assessment of sarcopenia and changes in body composition during neoadjuvant chemotherapy for esophageal cancer. Anticancer Res. 2017;37:3053–9. https://doi.org/10.21873/anticanres.11660.
Author information
Authors and Affiliations
Contributions
GFPA and NSM—conceptualization, data curation, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing the original draft and writing review and editing. WW—formal analysis and writing review and editing. P-HC and NSG—conceptualization, investigation, methodology, supervision, validation, visualization, writing to review and editing. FA, RD, BKH, BTH, DJ, JK, BP, RS, AW and PC—data curation, methodology, investigation, validation, visualization, writing to review and editing.
Corresponding author
Ethics declarations
Competing interests
None of the authors has any conflict of interest to declare in relation to this study. Outside the scope of this research, the following authors have financial conflicts of interest to report: NSM serves consultant for Anthem, Inc and own stock from HCA Healthcare.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Cleveland Clinic Consent to participate.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aleixo, G.F.P., Wei, W., Chen, PH. et al. The association of body composition and outcomes following autologous hematopoietic stem cell transplantation in patients with non-Hodgkin lymphoma. Bone Marrow Transplant 58, 1384–1389 (2023). https://doi.org/10.1038/s41409-023-02104-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02104-2