Abstract
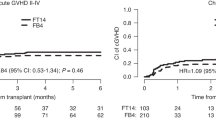
Different doses of treosulfan plus fludarabine have shown advantage over reduced intensity regimens. However, data comparing higher doses of treosulfan to myeloablative busulfan are limited. Thus, we compared outcomes between FT14 (fludarabine 150/160 mg/m2 and treosulfan 42 g/m2, or FT14) over FB4 (fludarabine 150/160 mg/m2 and busulfan 12.8 mg/kg). We retrospectively studied patients from European Society for Blood and Marrow Transplantation registry: a) adults diagnosed with acute myeloid leukemia (AML), b) recipients of first allogeneic hematopoietic stem cell transplantation (HSCT) from unrelated or sibling donor (2010–2020), c) HSCT at first or second complete remission, d) conditioning with FT14 or FB4. FT14 recipients (n = 678) were older, with higher rates of secondary AML, unrelated donors, peripheral blood grafts, and adverse cytogenetics, but lower percentage of female donor to male recipient compared to FB4 (n = 2025). Analysis was stratified on age. In patients aged < 55 years, FT14 was associated with higher relapse incidence (RI) and lower Leukemia-Free Survival (LFS). In patients aged≥55 years, acute GVHD CI was higher in FB4, without significant differences in other outcomes. Although FT14 has been used for higher-risk HSCT patients, our large real-world multicenter study suggests that FB4 is associated with better outcomes compared to FT14 in younger patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data are available upon request from the corresponding author.
References
Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer. 2013;119:2720–7. https://doi.org/10.1002/cncr.28129.
Alam N, Atenafu EG, Kuruvilla J, Uhm J, Lipton JH, Messner HA, et al. Outcomes of patients with therapy-related AML/myelodysplastic syndrome (t-AML/MDS) following hematopoietic cell transplantation. Bone marrow Transplant. 2015;50:1180–6. https://doi.org/10.1038/bmt.2015.151.
Kayser S, Dohner K, Krauter J, Kohne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood 2011;117:2137–45. https://doi.org/10.1182/blood-2010-08-301713.
Jethava YS, Sica S, Savani B, Socola F, Jagasia M, Mohty M, et al. Conditioning regimens for allogeneic hematopoietic stem cell transplants in acute myeloid leukemia. Bone marrow Transplant. 2017;52:1504–11. https://doi.org/10.1038/bmt.2017.83.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2009;15:1628–33. https://doi.org/10.1016/j.bbmt.2009.07.004.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–63.
de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–72. https://doi.org/10.1182/blood-2003-11-3750.
Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–8. https://doi.org/10.1038/sj.leu.2404037.
Oliansky DM, Appelbaum F, Cassileth PA, Keating A, Kerr J, Nieto Y, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myelogenous leukemia in adults: an evidence-based review. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2008;14:137–80. https://doi.org/10.1016/j.bbmt.2007.11.002.
Nagler A, Labopin M, Shimoni A, Niederwieser D, Mufti GJ, Zander AR, et al. Mobilized peripheral blood stem cells compared with bone marrow as the stem cell source for unrelated donor allogeneic transplantation with reduced-intensity conditioning in patients with acute myeloid leukemia in complete remission: an analysis from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2012;18:1422–9. https://doi.org/10.1016/j.bbmt.2012.02.013.
Danylesko I, Shimoni A, Nagler A. Treosulfan-based conditioning before hematopoietic SCT: More than a BU look-alike. Bone marrow Transplant. 2012;47:5–14. https://doi.org/10.1038/bmt.2011.88.
Slatter MA, Boztug H, Potschger U, Sykora KW, Lankester A, Yaniv I, et al. Treosulfan-based conditioning regimens for allogeneic haematopoietic stem cell transplantation in children with non-malignant diseases. Bone marrow Transplant. 2015;50:1536–41. https://doi.org/10.1038/bmt.2015.171.
Strocchio L, Zecca M, Comoli P, Mina T, Giorgiani G, Giraldi E, et al. Treosulfan-based conditioning regimen for -logeneic haematopoietic stem cell transplantation in children with sickle cell disease. Br urinal Haematol. 2015;169:726–36. https://doi.org/10.1111/bjh.13352.
Boztug H, Sykora KW, Slatter M, Zecca M, Veys P, Lankester A, et al. European Society for Blood and Marrow Transplantation Analysis of Treosulfan Conditioning Before Hematopoietic Stem Cell Transplantation in Children and Adolescents With Hematological Malignancies. Pediatr Blood Cancer. 2016;63:139–48. https://doi.org/10.1002/pbc.25764.
Ruutu T, Volin L, Beelen DW, Trenschel R, Finke J, Schnitzler M, et al. Reduced-toxicity conditioning with treosulfan and fludarabine in allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes: final results of an international prospective phase II trial. Haematologica. 2011;96:1344–1350. https://doi.org/10.3324/haematol.2011.043810.
Sjoo F, Hassan Z, Abedi-Valugerdi M, Griskevicius L, Nilsson C, Remberger M, et al. Myeloablative and immunosuppressive properties of treosulfan in mice. Exp Hematol. 2006;34:115–21. https://doi.org/10.1016/j.exphem.2005.09.015.
Glowka F, Kasprzyk A, Romanski M, Wrobel T, Wachowiak J, Szpecht D, et al. Pharmacokinetics of treosulfan and its active monoepoxide in pediatric patients after intravenous infusion of high-dose treosulfan prior to HSCT. Eur J Pharm Sci: Off J Eur Federation Pharm Sci. 2015;68:87–93. https://doi.org/10.1016/j.ejps.2014.12.010.
Kroger N, Shimoni A, Zabelina T, Schieder H, Panse J, Ayuk F. et al.Reduced-toxicity conditioning with treosulfan, fludarabine and ATG as preparative regimen for allogeneic stem cell transplantation (alloSCT) in elderly patients with secondary acute myeloid leukemia (sAML) or myelodysplastic syndrome (MDS).Bone Marrow Transpl.2006;37:339–44. https://doi.org/10.1038/sj.bmt.1705259.
Shimoni A, Hardan I, Shem-Tov N, Rand A, Yerushalmi R, Nagler A. Fludarabine and treosulfan: a novel modified myeloablative regimen for allogeneic hematopoietic stem-cell transplantation with effective antileukemia activity in patients with acute myeloid leukemia and myelodysplastic syndromes. Leuk Lymphoma. 2007;48:2352–9. https://doi.org/10.1080/10428190701671051.
Nemecek ER, Guthrie KA, Sorror ML, Wood BL, Doney KC, Hilger RA, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2011;17:341–50. https://doi.org/10.1016/j.bbmt.2010.05.007.
Casper J, Holowiecki J, Trenschel R, Wandt H, Schaefer-Eckart K, Ruutu T, et al. Allogeneic hematopoietic SCT in patients with AML following treosulfan/fludarabine conditioning. Bone marrow Transplant. 2012;47:1171–7. https://doi.org/10.1038/bmt.2011.242.
Gyurkocza B, Gutman J, Nemecek ER, Bar M, Milano F, Ramakrishnan A, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2014;20:549–55. https://doi.org/10.1016/j.bbmt.2014.01.009.
Casper J, Wolff D, Knauf W, Blau IW, Ruutu T, Volin L, et al. Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28:3344–51. https://doi.org/10.1200/JCO.2009.23.3429.
Zhu S, Liu G, Liu J, Chen Q, Wang Z. Long-Term Outcomes of Treosulfan- vs. Busulfan-Based Conditioning Regimen for Patients With Myelodysplastic Syndrome and Acute Myeloid Leukemia Before Hematopoietic Cell Transplantation: A Systematic Review and Meta-Analysis. Front Oncol. 2020;10:591363 https://doi.org/10.3389/fonc.2020.591363.
Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Remenyi, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): A randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020;7:e28–e39. https://doi.org/10.1016/S2352-3026(19)30157-7.
Sakellari I, Mallouri D, Gavriilaki E, Batsis I, Kaliou M, Constantinou V, et al. Survival Advantage and Comparable Toxicity in Reduced-Toxicity Treosulfan-Based versus Reduced-Intensity Busulfan-Based Conditioning Regimen in Myelodysplastic Syndrome and Acute Myeloid Leukemia Patients after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2017;23:445–51. https://doi.org/10.1016/j.bbmt.2016.11.023.
Shimoni A, Shem-Tov N, Volchek Y, Danylesko I, Yerushalmi R, Nagler A. Allo-SCT for AML and MDS with treosulfan compared with BU-based regimens: reduced toxicity vs reduced intensity. Bone marrow Transplant. 2012;47:1274–82. https://doi.org/10.1038/bmt.2012.4.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706.
Sakellari I, Gavriilaki E, Mallouri D, Batsis I, Varelas C, Tagara S, et al. Survival Advantage of Treosulfan Plus Fludarabine Before Allogeneic Hematopoietic Cell Transplantation for Older or Comorbid Patients With Myeloid Malignancies. Transpl Cell Ther. 2021;27:916 e911–916. https://doi.org/10.1016/j.jtct.2021.07.020.
Passweg JR, Baldomero H, Basak GW, Chabannon C, Corbacioglu S, Duarte R, et al. The EBMT activity survey report 2017: A focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transplant. 2019;54:1575–85. https://doi.org/10.1038/s41409-019-0465-9.
Loke J, Malladi R, Moss P, Craddock C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: A triumph of hope and experience. Br J Haematol. 2020;188:129–46. https://doi.org/10.1111/bjh.16355.
Fasslrinner F, Schetelig J, Burchert A, Kramer M, Trenschel R, Hegenbart U, et al. Long-term efficacy of reduced-intensity versus myeloablative conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: Retrospective follow-up of an open-label, randomised phase 3 trial. Lancet Haematol. 2018;5:e161–e169. https://doi.org/10.1016/S2352-3026(18)30022-X.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol: Off J Am Soc Clin Oncol. 2017;35:1154–61. https://doi.org/10.1200/JCO.2016.70.7091.
Rambaldi A, Grassi A, Masciulli A, Boschini C, Mico MC, Busca A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2015;16:1525–36. https://doi.org/10.1016/S1470-2045(15)00200-4.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: A study in acute myeloid leukemia patients. Bone Marrow Transplant. 2020;55:1114–25. https://doi.org/10.1038/s41409-020-0803-y.
Wedge E, Sengelov H, Hansen JW, Andersen NS, Schjodt I, Petersen SL, et al. Improved Outcomes after Allogenic Hematopoietic Stem Cell Transplantation with Fludarabine/Treosulfan for Patients with Myelodysplastic Syndromes. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2020;26:1091–8. https://doi.org/10.1016/j.bbmt.2020.02.010.
Fein JA, Shimoni A, Labopin M, Shem-Tov N, Yerushalmi R, Magen H, et al. The impact of individual comorbidities on non-relapse mortality following allogeneic hematopoietic stem cell transplantation. Leukemia 2018;32:1787–94. https://doi.org/10.1038/s41375-018-0185-y.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9. https://doi.org/10.1182/blood-2005-05-2004.
Cheung E, Perissinotti AJ, Bixby DL, Burke PW, Pettit KM, Benitez LL, et al. The leukemia strikes back: A review of pathogenesis and treatment of secondary AML. Ann Hematol. 2019;98:541–59. https://doi.org/10.1007/s00277-019-03606-0.
Sengsayadeth S, Labopin M, Boumendil A, Finke J, Ganser A, Stelljes M, et al. Transplant Outcomes for Secondary Acute Myeloid Leukemia: Acute Leukemia Working Party of the European Society for Blood and Bone Marrow Transplantation Study. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2018;24:1406–14. https://doi.org/10.1016/j.bbmt.2018.04.008.
Acknowledgements
EG is supported by the ASH Global Research Award.
Author information
Authors and Affiliations
Contributions
EG and IS designed research and wrote the original draft, provided clinical data; ML participated in research design, performed statistical analysis, and drafted the Tables and Figures; US, IYA, VP, AB, AR, IH, NK, SM, TZ, JS, AB, HO provided clinical data and edited the manuscript; AA, BS, SG, AB, AS, AN, and MM participated in research design and edited the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gavriilaki, E., Labopin, M., Sakellari, I. et al. Comparative study of treosulfan plus Fludarabine (FT14) with busulfan plus Fludarabine (FB4) for acute myeloid leukemia in first or second complete remission: An analysis from the European Society for Blood and Marrow Transplantation (EBMT) Acute Leukemia Working Party (ALWP). Bone Marrow Transplant 57, 1803–1809 (2022). https://doi.org/10.1038/s41409-022-01830-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01830-3
This article is cited by
-
Fludarabine-treosulfan versus fludarabine-melphalan or busulfan-cyclophosphamide conditioning in older AML or MDS patients – A clinical trial to registry data comparison
Bone Marrow Transplantation (2024)
-
Treosulfan compared to busulfan in allogeneic haematopoietic stem cell transplantation for myelofibrosis: a registry-based study from the Chronic Malignancies Working Party of the EBMT
Bone Marrow Transplantation (2024)
-
Validation of the transplant conditioning intensity (TCI) index for allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2024)