Abstract
The optimal reduced intensity conditioning (RIC) regimen is a matter of debate. We retrospectively compared conditioning with fludarabine plus fractionated total body irradiation of 8 Gy (FluTBI) and fludarabine plus treosulfan 30, 36 or 42 g/m2 (FluTreo) in 754 patients with AML above the age of 40 years undergoing an allogeneic hematopoietic stem cell transplant (HSCT) in first complete remission (CR). After balancing patient characteristics by propensity score matching of 115 patients in each group, FluTBI was associated with a significantly lower probability of relapse compared to FluTreo (18.3% vs. 34.7%, p = 0.018) which was counteracted by a higher non-relapse mortality (NRM, 16.8% vs. 5.3%, p = 0.02). Thus, overall survival and graft-versus-host disease-free and relapse-free survival at 2 years were similar between groups (OS 66.9% vs. 67.8%, GRFS 50.3% vs. 45.6%). Univariate analysis by age group demonstrated a higher NRM exclusively in patients ≥55 years of age treated with FluTBI compared to FluTreo (27.6% vs. 5.8%, p = 0.02), while a similarly low NRM was observed in patients <55 years in both groups (6.0% vs. 4.7%, p = ns). We conclude that both conditioning regimens are effective and safe, but FluTBI may better be reserved for younger patients below the age of 55 years.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) in first complete remission (CR) is the treatment of choice for the majority of patients with acute myeloid leukemia (AML) [1,2,3], but its antileukemic efficacy needs to be balanced against the risk of non-relapse morbidity and mortality [4]. To address the higher non-relapse mortality (NRM) associated with increasing age, especially with myeloablative conditioning (MAC) regimens, reduced intensity conditioning (RIC) has been widely adopted [5]. Unfortunately, RIC regimens may result in higher relapse rates especially in patients with measurable residual disease (MRD) as demonstrated in the large randomized BMT CTN 0901 trial [6, 7]. This trial compared a busulfan- or total body irradiation (TBI)-based MAC regimen to RIC consisting of fludarabine and an alkylating agent (i.e. IV busulfan 6.4 mg/kg, FB2 or melphalan ≤150 mg/m2, FluMel) in adults patients up to the age of 65 years with AML or myelodysplastic syndrome (MDS) and <5% bone marrow blasts at HSCT. Several efforts have been made to overcome the hurdle of insufficient antileukemic activity of RIC as well as excess tocixity of MAC regimens.
FB2 was challenged by fludarabine and treosulfan (FluTreo) in patients with AML in CR or MDS at increased risk of mortality with MAC. Treosulfan is a prodrug of a bifunctional alkylating agent with stem cell depleting and broad antileukemic activity and gained approval in the EU and Canada as part of conditioning based on a pivotal randomized trial in which FluTreo (30 g/m2 total dose) resulted in improved relapse-free survival (RFS) and OS (64% and 71% vs. 50% and 56% at 2 years, respectively), mainly due to reduction of late NRM from 22% to 11% [8]. In a randomized comparison of TBI-based MAC (i.e., fractionated TBI of 12 Gy and cyclophosphamide, TBI 12 Gy/Cy) with a reduced-toxicity conditioning (RTC) regimen of fludarabine and fractionated TBI of 8 Gy in AML in first CR (CR1), patients with AML aged 41 to 60 years treated with RTC achieved favorable RFS of 76% at 1 year and a sustained low NRM of 13% at 10 years [9, 10].
To date, no direct comparison of fludarabine and treosulfan (FluTreo) with fludarabine and TBI 8 Gy (FluTBI) has been performed. We hypothesize that FluTreo may be a lower toxicity alternative to the FluTBI 8 Gy regimen in AML patients aged above 40 years in CR1, who may not be prime candidates for MAC regimens.
Methods
Data collection
Data for this retrospective multicenter study were retrieved from the registry of the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT), a nonprofit, scientific society representing >600 transplant centers, mainly located in Europe. Centers commit to reporting all consecutive HSCT and follow-ups once a year. Data are entered, managed, and maintained in a central database and validated by verification of the computer printout of the entered data, cross-checking with the national registries, and on-site visits to selected teams. All patients gave informed consent authorizing the use of their personal information for research purposes. This study was approved by the ALWP of the EBMT institutional review board and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Criteria for patient selection
Patients were included if they had (1) a diagnosis of AML in CR1 (MRD positive or negative); (2) an age >40 years; (3) received their first allogeneic HSCT between 2009 and 2019; (4) a matched sibling donor (MSD) or 10/10 HLA-matched unrelated donor (MUD), and if (5) peripheral blood stem cells or bone marrow was used as the stem cell graft; (6) the conditioning regimen consisted of either fludarabine and treosulfan (30, 36 or 42 g/m2, FluTreo) or fludarabine and fractionated TBI 8 Gy (4 × 2 Gy or 2 × 4 Gy, FluTBI). In vivo T- cell depletion (TCD) with anti-thymocyte globulin (ATG) was allowed, but transplantations from haploidentical donors, umbilical cord blood stem cells or using post-transplant cyclophosphamide or ex-vivo T-cell depletion were excluded. Transplant centers were asked to report MRD status at time of HSCT.
Statistical analysis
The primary endpoint of this study was OS; secondary endpoints included LFS, cumulative incidence of relapse (CIR), NRM, incidence of acute and chronic graft-versus-host disease (GVHD) as well as survival free of grade III-IV acute GVHD or severe chronic GVHD (GRFS) [11]. Acute and chronic GVHD were diagnosed according to the modified Glucksberg criteria and modified Seattle criteria, respectively [12, 13].
Patient, disease, and transplant characteristics were compared by using the χ2 or Fisher’s exact test for categorial variables and the Mann-Whitney or Kruskal Wallis test for continuous variables. Probabilities for OS, LFS and GRFS were calculated using Kaplan-Meier estimates, and cumulative incidence (CI) curves for relapse, NRM, acute and chronic GVHD using a competing risk model: relapse and death are competing together, i.e. relapse is the competing event for NRM and death without relapse the competing event for relapse, whereas relapse and death were competing risks for GVHD [14]. Univariate analyses were performed using the log-rank test for LFS, OS, and GRFS, and Gray’s test for CI estimates [15].
For propensity score matching, exact matching was performed in a 1:1 ratio for donor type, secondary AML and adverse risk cytogenetics and nearest neighbor matching for age at HSCT, time from diagnosis to HSCT, female to male transplant, Karnofsky performance score (KPS) and in vivo TCD. We compared 115 patients in each conditioning group. All tests were two-sided with the type 1 error rate fixed at 0.05. SPSS 27.0 (IBM Corp., Armonk, NY, USA) and R 4.0.2 (R Core Team 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.Rproject.org/), were used for all statistical analyses.
Results
Patients and transplant procedures
A total of 754 patients with AML are included in this analysis, of whom 617 received FluTreo and 137 FluTBI conditioning. Patients in the FluTBI group were significantly younger with a median age of 53.7 (range, 40.1–70.7) vs. 60.7 (range 40.1–77.5) years. They had been transplanted a median of two years earlier (2014 vs. 2016) and had longer follow-up (median 45.9 vs. 26.9 months), Table 1. Additional statistically significant differences between the groups included a higher proportion of MSD with FluTBI (64.2% vs. 36.3%, p < 0.0001) as opposed to 10/10 MUD (35.8% vs. 63.7%, p < 0.0001), a shorter time from diagnosis to HSCT (3.8 (range, 1.8–16.2) vs. 4.7 (range, 1.7–22.9 months)) and a higher proportion of patients with de novo AML (84.7% vs. 76%). The groups did not differ significantly in terms of adverse risk cytogenetics according to European LeukemiaNet (ELN) 2017, patient or donor sex, KPS, or pre-transplant MRD status, although information on the latter parameter was available for only 287 patients.
The major difference in relation to transplant procedures was the significantly more frequent use of in vivo TCD with ATG in the FluTreo group (67.3% vs. 46%, p < 0.0001), Table 1.
Pair-match analysis on propensity score
Because of the substantial difference in patient numbers between the two conditioning groups and significant differences in demographic and transplant-related parameters, we used propensity score matching to reduce the treatment assignment bias and create two patient groups of 115 each that were comparable for all observed covariates. Patient characteristics in the FluTBI and FluTreo group were well balanced in terms of age (median 55.2 vs. 54.9 years, KPS < 90% 22.6% and 23.5%, respectively), secondary AML (13% each), adverse cytogenetics (15.7% each), female donor to male recipient (19.1% vs. 17.4%) and time from diagnosis to HSCT (median 3.8 (range, 1.8–16.2) and 4.5 (range, 1.7–16.2) months, respectively), Table 2. An identical proportion of patients in both groups received grafts from MSD (61.7%) or 10/10 HLA-MUD (38.3%). In both groups, GVHD prophylaxis consisted predominantly of cyclosporin A (CSA) plus methotrexate (85.2% for FluTBI vs. 73.9% for FluTreo, p=ns), CSA and mycophenolate mofetil were given to 8.7% and 18.3% of patients in the FluTBI and FluTreo groups, respectively (p=ns). A similar proportion of patients in both groups (52.2% and 53.9%) received additional in vivo T-cell depletion with ATG.
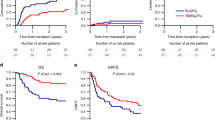
All but one patient in each group engrafted. Median follow-up of living patients was 42.4 months (range, 31.5–53.8) in the FluTBI and 23.2 months (range, 20.4–32.7) in the FluTreo group (p = 0.14). FluTBI was associated with a significantly lower CIR of 18.3% vs. 34.7% with FluTreo (p = 0.018, HR 0.51 (95% CI, 0.29–0.89)), but a higher NRM of 16.8% vs. 5.3%, p = 0.02, HR 3.0 (95% CI, 1.19–7.59), Fig. 1a, b. This difference in NRM was due exclusively to the higher NRM in patients ≥55 years of age (Table 3). LFS and OS were similar in the FluTBI and FluTreo groups (64.9% vs. 60.0%, HR 0.84 (95% CI, 0.54–1.31) and 66.9% vs. 67.8%, HR 1.08 (95% CI, 0.67–1.75)), respectively, Fig. 1c, d. Infection was the leading cause of death following FluTBI (n = 12, 34.3% vs. n = 3, 9.4% with FluTreo), whereas AML recurrence was the predominant cause of death in the FluTreo group (n = 15, 46.9% vs. n = 10, 28.6%). The frequency of death due to GVHD, multiorgan failure or interstitial pneumonitis did not differ between the two groups. Two patients developed a secondary malignancy after TBI conditioning, results not shown.
There was no statistically significant difference between FluTBI and FluTreo in the CI of acute GVHD II-IV (22.8% vs. 20.7%, HR 1.05), GVHD III-IV (6.2% vs. 9.0%, HR 0.59), chronic GVHD (42.6% vs. 47.5%, HR 0.81) or extensive chronic GVHD (16.8% vs. 19.6%, HR 0.76), results not shown, resulting in similar GRFS of 50.3% and 45.6%, HR 0.83, respectively (Fig. 1e).
Discussion
Because of its manageable extramedullary toxicity profile and satisfactory anti-leukemic activity in a randomized registration study, the combination of fludarabine and treosulfan 30 g/m2 has been increasingly adopted as the RIC of choice in patients with AML and MDS who are ineligible for MAC [8]. In a large retrospective study, this conditioning regimen using higher treosulfan doses was shown to be tolerable and effective in patients with more advanced AML and a median age of 57 years [16]. We hypothesized that FluTreo might be an alternative to the RTC FluTBI 8 Gy in patients above 40 years with AML in CR1, for whom favorable long-term outcomes have recently been reported in a randomized comparison with TBI 12 Gy/Cy MAC [9, 10]. Our study demonstrates that FluTBI conditioning prior to 10/10 HLA matched allogeneic HSCT achieves good leukemic control in this patient population with a low relapse rate of 18.3% and modest NRM of 16.8%. The probabilities of LFS and OS at two years (64.9% and 66.9%, respectively) match the outcome data reported for the subgroup of patients aged 41–60 years in the FluTBI 8 Gy arm of the German MAC vs. RTC trial.
Our hypothesis that the antileukemic efficacy of FluTreo would be equivalent to that of FluTBI was not borne out by the results of our pair-match analysis, which demonstrated a significantly higher CIR with FluTreo compared with FluTBI conditioning. Moreover, the relapse rate of 34.7% in our FluTreo cohort was higher than in the FluTreo arm (24.6%) of the randomized registration trial [8]. This may be attributable to differences in the patient population, as the latter study included not only AML, but also 30% of MDS patients, with MDS patients experiencing fewer relapses (Supplementary Appendix [8],). In addition, a larger proportion of patients in the randomized study developed chronic, and in particular mild chronic GVHD, which may have contributed to the lower relapse rate. As chronic GVHD did not significantly contribute to mortality in either study, the lower incidence of chronic GVHD seen in our analysis is consistent with the higher observed CIR. In vivo TCD was included in the propensity score for pair-matching and its distribution well balanced between the two conditioning regimens (47.8% vs 46.1% in FluTBI and FluTreo groups, respectively). Consequently, it cannot explain the higher risk of relapse in the FluTreo group.
An additional possibility is that differences in the treosulfan dose may have contributed to the unexpectedly high relapse rate although there is no conclusive evidence of greater antileukemic efficacy of higher treosulfan doses. NRM however proved to be higher with 42 g/m2 compared to the 30 g/m2 dose in the pivotal randomized study which was stopped after an interim analysis had shown prolonged neutropenia and subsequent serious infectious complications with fludarabine and treosulfan 42 g/m2 total dose compared to the RIC regimen FB2. The protocol was amended to a reduced treosulfan dose of 30 g/m2 and demonstrated superior overall and relapse-free survival of patients in the FluTreo arm [8, 17]. In our study, NRM was low despite most patients receiving treosulfan doses higher than 30 g/m2.
Comparing the patient cohort in our analysis and the randomized study evaluating FluTBI 8 Gy, NRM in the FluTBI group in our study seemed to be somewhat higher, with the caveat of a different duration of follow-up (16.8% at 2 years vs. 13.0% at 10 years). We show that a 55 year age threshold discriminates between patients with low and high NRM, even though this does not translate into inferior OS and RFS in the older age cohort. This discrepancy may be due to the fact that the lower relapse rate with FluTBI did not reach statistical significance given the relatively small number of patients. Nevertheless, it appears advisable to employ FluTBI with considerable caution in patients 55 years and above. Another possible explanation for this difference in NRM is the TBI schedule used in these two studies: whereas the randomized study consistently administered TBI in four fractions of 2 Gy, our analysis also included patients in whom 8 Gy TBI was delivered in 2 fractions of 4 Gy. A retrospective study comparing delivery of 12 Gy TBI in one or two fractions over 3 days suggested a higher risk of organ toxicity, but not NRM with the 1-day fractionation [18]. Additional confounding variables might have been introduced by the heterogeneity of TBI techniques used in different centers [19] and/or by center preferences in the application of only FluTBI (8 centers), only FluTreo (52 centers) or both (15 centers). However, we found no such center effects (data not shown) [20].
In addition to age, the level of MRD at the time of HSCT is a well-known determinant of relapse rate and outcome. There was no significant difference in the proportion of MRD positive and negative patients in the two conditioning groups in our study. However, MRD levels at transplant were available for only a minority of patients and techniques of MRD detection heterogeneous among centers [21], which is a limitation of the present analysis. After HSCT, incomplete T cell donor chimerism may identify AML patients at high risk for disease recurrence, but this information has not been captured in the EBMT registry [22].
Taken together, our retrospective analysis demonstrates that the FluTBI and FluTreo conditioning regimens result in comparable survival in patients with AML undergoing HSCT in CR1. In view of the randomized BMT CTN 0901 trial, which was reported after the time period encompassing our present analysis, centers may prefer a MAC regimen including TBI 12 Gy or busulfan 12.8 mg/kg for patients who proceed to HSCT with MRD positive AML [6, 7]. However, at least the RTC FluTBI evaluated in our study is more intensive than the RIC regimens explored in the BMT CTN 0901 trial [23], and our study furthermore includes a separate analysis of NRM in patients <55 and ≥55 years of age. In the latter patients, the more intensive FluTBI regimen was associated with a significantly higher NRM, whereas the age-dependency of NRM was not analysed separately in the BMT CTN 0901 trial. Although we did not identify patient subgroups who derived significant benefit from the enhanced antileukemic activity of FluTBI, patients <55 years of age with high-risk leukemias and a low HCT-specific comorbidity index (HCT-CI) may do better with FluTBI. Robust long-term outcome data consistent with this concept have been reported [10].
NRM with FluTreo was remarkably low even in patients at higher risk of toxic death and could likely be the preferred type of conditioning for patients with such risk features [5]. It will also be of interest to determine whether the more user friendly chemotherapy based FluTreo regimen may have fewer long-term side effects than TBI based conditioning, provided outcome is the same. A similarly favorable NRM was reported in a randomized trial for the widely used RIC regimen fludarabine and TBI 2 Gy although for the price of a less efficacious disease control in a variety of hematologic diseases compared to FB2 [24]. In an effort to further enhance the antileukemic activity of FB2, the augmented conditioning regimen FLAMSA-Bu was applied to patients with high-risk AML in CR1, CR2 or with primary refractory disease in the randomized FIGARO trial but failed to improve relapse rates compared to the RIC FluMel [25].
Our results strongly suggest that strategies that build on the excellent tolerability of FluTreo and focus on reducing the higher relapse rate associated with this regimen conceptually have promise. Recently, O´Hagan Henderson et al. demonstrated the feasibility of fludarabine and treosulfan 42 g/m2 in combination with high-dose cytarabine in 77 patients with poor-risk myeloid neoplasms, 54% of whom were not in CR. In the subgroup of 58 AML patients, OS and CIR at 3 years were 44% and 43%, respectively [26]. The combination of FluTreo with TBI 2 Gy has been pioneered by Gyurkocza et al. in AML and MDS patients [27], analogous to the successful sequential conditioning regimen of fludarabine and melphalan followed by TBI 8 Gy in relapsed and refractory AML [28]. Addition of low-dose TBI to FluTreo was associated with considerable gastrointestinal toxicity but nevertheless a low NRM of 8% and a CIR of 27% at 2 years [27]. Conspicuously, this approach did not appear to mitigate the high post-transplant relapse rate in patients with MRD pre-HSCT as opposed to patients who were MRD negative (CIR 70% and 18%, respectively). This highlights the need to also explore additional approaches such as post-transplant maintenance strategies and more effective pre-transplant therapies.
Data availability
Upon request to the ALWP of the EBMT (Dr Myriam Labopin).
References
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Bejanyan N, Zhang M, Bo-Subait K, Brunstein C, Wang H, Warlick ED, et al. Myeloablative conditioning for allogeneic transplantation results in superior disease-free survival for acute myelogenous leukemia and myelodysplastic syndromes with low/intermediate but not high disease risk index: a center for international blood and marrow transplant research study. Transpl Cell Ther. 2021;27:68.e1–e9.
Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Camara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transpl. 2021;56:1651–64.
Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127:62–70.
Saraceni F, Scortechini I, Fiorentini A, Dubbini MV, Mancini G, Federici I, et al. Conditioning regimens for frail patients with acute leukemia undergoing allogeneic stem cell transplant: how to strike gently. Clin Hematol Int. 2021;3:153–60.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–61.
Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. 2020;38:1273–83.
Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Remenyi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020;7:e28–e39.
Bornhauser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–44.
Fasslrinner F, Schetelig J, Burchert A, Kramer M, Trenschel R, Hegenbart U, et al. Long-term efficacy of reduced-intensity versus myeloablative conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: retrospective follow-up of an open-label, randomised phase 3 trial. Lancet Haematol. 2018;5:e161–e9.
Kanate AS, Nagler A, Savani B. Summary of scientific and statistical methods, study endpoints and definitions for observational and registry-based studies in hematopoietic cell transplantation. Clin Hematol Int. 2020;2:2–4.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow Transplant. 1995;15:825–8.
Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2003;9:215–33.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Fine J, Gray R. A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Nagler A, Labopin M, Beelen D, Ciceri F, Volin L, Shimoni A, et al. Long-term outcome after a treosulfan-based conditioning regimen for patients with acute myeloid leukemia: A report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer. 2017;123:2671–9.
Clinical Study Report. FInal Analysis of 330 Patients included into the clinical study of MC-FludT14.L before Amendment No. 03 was effective [Internet]. 2019 [cited 6th February 2022]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2008-002356-18/results.
Belkacemi Y, Labopin M, Giebel S, Loganadane G, Miszczyk L, Michallet M, et al. Single-Dose Daily Fractionation Is Not Inferior to Twice-a-Day Fractionated Total-Body Irradiation Before Allogeneic Stem Cell Transplantation for Acute Leukemia: A Useful Practice Simplification Resulting From the SARASIN Study. Int J Radiat Oncol Biol Phys. 2018;102:515–26.
Giebel S, Miszczyk L, Slosarek K, Moukhtari L, Ciceri F, Esteve J, et al. Extreme heterogeneity of myeloablative total body irradiation techniques in clinical practice: a survey of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2014;120:2760–5.
Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–500.
Nagler A, Baron F, Labopin M, Polge E, Esteve J, Bazarbachi A, et al. Measurable residual disease (MRD) testing for acute leukemia in EBMT transplant centers: a survey on behalf of the ALWP of the EBMT. Bone marrow Transpl. 2021;56:218–24.
Lee HC, Saliba RM, Rondon G, Chen J, Charafeddine Y, Medeiros LJ, et al. Mixed T lymphocyte chimerism after allogeneic hematopoietic transplantation is predictive for relapse of acute myeloid leukemia and myelodysplastic syndromes. Biol Blood Marrow Transpl. 2015;21:1948–54.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55:1114–25.
Blaise D, Tabrizi R, Boher JM, Le Corroller-Soriano AG, Bay JO, Fegueux N, et al. Randomized study of 2 reduced-intensity conditioning strategies for human leukocyte antigen-matched, related allogeneic peripheral blood stem cell transplantation: prospective clinical and socioeconomic evaluation. Cancer. 2013;119:602–11.
Craddock C, Jackson A, Loke J, Siddique S, Hodgkinson A, Mason J, et al. Augmented reduced-intensity regimen does not improve postallogeneic transplant outcomes in acute myeloid leukemia. J Clin Oncol. 2021;39:768–78.
O’Hagan Henderson S, Frietsch JJ, Hilgendorf I, Hochhaus A, Kohne CH, Casper J Combination of treosulfan, fludarabine and cytarabine as conditioning in patients with acute myeloid leukemia, myelodysplastic syndrome and myeloproliferative neoplasms. J Cancer Res Clin Oncol. 2021;148:2599–609.
Gyurkocza B, Gutman J, Nemecek ER, Bar M, Milano F, Ramakrishnan A, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biol Blood Marrow Transpl. 2014;20:549–55.
Steckel NK, Groth C, Mikesch JH, Trenschel R, Ottinger H, Kordelas L, et al. High-dose melphalan-based sequential conditioning chemotherapy followed by allogeneic haematopoietic stem cell transplantation in adult patients with relapsed or refractory acute myeloid leukaemia. Br J Haematol. 2018;180:840–53.
Acknowledgements
We sincerely thank all the European Society for Blood and Marrow Transplantation (EBMT) centers (Supplementary data S1: Principal investigators of the contributing institutions) and national registries for contributing patients to this study; we also thank the data managers and the patients who contributed their data.
Funding
This work was supported by the Universitätsmedizin Essen Clinician Scientist Academy (UMEA). This work was further funded through the German-Israeli Foundation for Research and Development (I-65-412.20-2016 to HCR), the Deutsche Forschungsgemeinschaft (RE 2246/13-1 to HCR), the Deutsche Jose Carreras Leukämie Stiftung (R12/08 to HCR), the Deutsche Krebshilfe (1117240 and 70113041 to HCR), as well as the German Ministry of Education and Research (BMBF e:Med 01ZX1303A to HCR). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
GB designed the study and wrote the manuscript, ML performed the statistical analysis. AB, AS, BNS, AN and MM revised the manuscript and all the authors reviewed and accepted its final version. RN, MS, HCR, IH, NK, AK, WB, KSE, MV, SM and KC were the principal investigators at the centers recruiting the highest number of patients for the study. Members of the contributing institutions are listed as Supplementary Information.
Corresponding author
Ethics declarations
Competing interests
GB received research support from Novartis and honoraria and travel expenses from Jazz, Celgene, Novartis, and Gilead. HCR received consulting and lecture fees from Abbvie, AstraZeneca, Vertex, Novartis and Merck and research funding from Gilead Pharmaceuticals and AstraZeneca. HCR is a co-founder of CDL Therapeutics GmbH. IH received honoraria from Novartis, ABBVIE, has obtained travel, accommodation or expenses from medac GmbH, Janssen-Ciliag, Jazz Pharmaceuticals and Celgene. WB received honoraria from Miltenyi, Celgene, Gilead und Novartis and travel exprenses from Medac. All other authors declare no conflict of interest.
Ethics approval
This study was approved by the ALWP of the EBMT institutional review board and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Patient consent statement
All patients gave informed consent authorizing the use of their personal information for research purposes.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bug, G., Labopin, M., Niittyvuopio, R. et al. Fludarabine/TBI 8 Gy versus fludarabine/treosulfan conditioning in patients with AML in first complete remission: a study from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant 58, 710–716 (2023). https://doi.org/10.1038/s41409-023-01965-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-01965-x