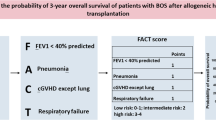
Abstract
Bronchiolitis obliterans syndrome (BOS) is the most morbid form of chronic graft-versus-host disease (cGVHD) after hematopoietic cell transplantation (HCT). Progressive airway fibrosis leads to a 5-year survival of 40%. Treatment options for BOS are limited. A single arm, 52-week, Phase I study of pirfenidone was conducted. The primary outcome was tolerability defined as maintaining the recommended dose of pirfenidone (2403 mg/day) without a dose reduction totaling more than 21 days, due to adverse events (AEs) or severe AEs (SAEs). Secondary outcomes included pulmonary function tests (PFTs) and patient reported outcomes (PROs). Among 22 participants treated for 1 year, 13 (59%) tolerated the recommended dose, with an average daily tolerated dose of 2325.6 mg/day. Twenty-two SAEs were observed, with 90.9% related to infections, none were attributed to pirfenidone. There was an increase in the average percent predicted forced expiratory volume in 1 s (FEV1%) of 7 percentage points annually and improvements in PROs related to symptoms of cGVHD. In this Phase I study, treatment with pirfenidone was safe. The stabilization in PFTs and improvements in PROs suggest the potential of pirfenidone for BOS treatment and support the value of a randomized controlled trial to evaluate the efficacy of pirfenidone in BOS after HCT. The study is registered in ClinicalTrials.gov (NCT03315741).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Additional study data generated for the current study are available from the corresponding author on reasonable request.
References
Williams KM. How I treat bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Blood. 2017;129:448–55. https://doi.org/10.1182/blood-2016-08-693507
Bergeron A, Godet C, Chevret S, Lorillon G, Peffault de Latour R, de Revel T, et al. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant. 2013;48:819–24. https://doi.org/10.1038/bmt.2012.241
Cheng GS, Storer B, Chien JW, Jagasia M, Hubbard JJ, Burns L, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc. 2016;13:1932–9. https://doi.org/10.1513/AnnalsATS.201604-262OC
Bergeron A, Chevret S, Chagnon K, Godet C, Bergot E, Peffault de Latour R, et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191:1242–9. https://doi.org/10.1164/rccm.201410-1818OC
King TE Jr., Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. https://doi.org/10.1056/NEJMoa1402582
Shah PV, Balani P, Lopez AR, Nobleza CMN, Siddiqui M, Khan S. A review of pirfenidone as an anti-fibrotic in idiopathic pulmonary fibrosis and its probable role in other diseases. Cureus. 2021;13:e12482 https://doi.org/10.7759/cureus.12482
Du J, Paz K, Flynn R, Vulic A, Robinson TM, Lineburg KE, et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-beta production. Blood. 2017;129:2570–80. https://doi.org/10.1182/blood-2017-01-758854
Hostettler Haack KE, Halter J, Tamm M. Pirfenidone treatment in patients with bronchiolitis obliterans syndrome following allogeneic hematopoietic stem cell transplantation. Eur Respir J. 2016;48:PA3923 https://doi.org/10.1183/13993003.congress-2016.PA3923
Ihle F, von Wulffen W, Neurohr C. Pirfenidone: a potential therapy for progressive lung allograft dysfunction? J Heart Lung Transplant. 2013;32:574–5. https://doi.org/10.1016/j.healun.2013.02.004
Vos R, Verleden SE, Ruttens D, Vandermeulen E, Yserbyt J, Dupont LJ, et al. Pirfenidone: a potential new therapy for restrictive allograft syndrome? Am J Transplant. 2013;13:3035–40. https://doi.org/10.1111/ajt.12474
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401 e381. https://doi.org/10.1016/j.bbmt.2014.12.001
Williams KM, Cheng GS, Pusic I, Jagasia M, Burns L, Ho VT, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:710–6. https://doi.org/10.1016/j.bbmt.2015.10.009
Kupferberg DH, Kaplan RM, Slymen DJ, Ries AL. Minimal clinically important difference for the UCSD Shortness of Breath Questionnaire. J Cardiopulm Rehabil. 2005;25:370–7. https://doi.org/10.1097/00008483-200511000-00011
Lee S, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:444–52. https://doi.org/10.1053/bbmt.2002.v8.pm12234170
Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–4. https://doi.org/10.1136/bmj.305.6846.160
Swigris JJ, Brown KK, Behr J, du Bois RM, King TE, Raghu G, et al. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med. 2010;104:296–304. https://doi.org/10.1016/j.rmed.2009.09.006
Teh C, Onstad L, Lee SJ. Reliability and validity of the modified 7-day lee chronic graft-versus-host disease symptom scale. Biol Blood Marrow Transplant. 2020;26:562–7. https://doi.org/10.1016/j.bbmt.2019.11.020
Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. https://doi.org/10.1164/ajrccm.159.1.9712108
Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE. 2014;9:e95192 https://doi.org/10.1371/journal.pone.0095192
Neighbors M, Cabanski CR, Ramalingam TR, Sheng XR, Tew GW, Gu C, et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respiratory Med. 2018;6:615–26. https://doi.org/10.1016/S2213-2600(18)30185-1
Li Z, Xie W, Liu T. Efficient feature selection and classification for microarray data. PLoS ONE. 2018;13:e0202167 https://doi.org/10.1371/journal.pone.0202167
Christin C, Hoefsloot HC, Smilde AK, Hoekman B, Suits F, Bischoff R, et al. A critical assessment of feature selection methods for biomarker discovery in clinical proteomics. Mol Cell Proteom. 2013;12:263–76. https://doi.org/10.1074/mcp.M112.022566
Cottin, V, Koschel, D, Gunther, A, Albera, C, Azuma, A, Skold, CM, et al. Long-term safety of pirfenidone: results of the prospective, observational PASSPORT study. ERJ Open Res. 4. https://doi.org/10.1183/23120541.00084-2018 (2018).
Akpek G, Chinratanalab W, Lee LA, Torbenson M, Hallick JP, Anders V, et al. Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol Blood Marrow Transplant. 2003;9:46–51. https://doi.org/10.1053/bbmt.2003.49999
Im A, Mitchell SA, Steinberg SM, Curtis L, Berger A, Baird K, et al. Prevalence and determinants of fatigue in patients with moderate to severe chronic GvHD. Bone Marrow Transplant. 2016;51:705–12. https://doi.org/10.1038/bmt.2015.320
Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Adverse events of pirfenidone for the treatment of pulmonary fibrosis: a meta-analysis of randomized controlled trials. PLoS ONE. 2012;7:e47024 https://doi.org/10.1371/journal.pone.0047024
Poo JL, Torre A, Aguilar-Ramirez JR, Cruz M, Mejia-Cuan L, Cerda E, et al. Benefits of prolonged-release pirfenidone plus standard of care treatment in patients with advanced liver fibrosis: PROMETEO study. Hepatol Int. 2020;14:817–27. https://doi.org/10.1007/s12072-020-10069-3
Glassberg MK, Wijsenbeek MS, Gilberg F, Petzinger U, Kirchgaessler KU, Albera, C. Effect of pirfenidone on breathlessness in patients with idiopathic pulmonary fibrosis. Eur Respir J. 54. https://doi.org/10.1183/13993003.00399-2019 (2019).
Khanum B, Guha R, Sur VP, Nandi S, Basak SK, Konar A, et al. Pirfenidone inhibits post-traumatic proliferative vitreoretinopathy. Eye. 2017;31:1317–28. https://doi.org/10.1038/eye.2017.21
Nakayama S, Mukae H, Sakamoto N, Kakugawa T, Yoshioka S, Soda H, et al. Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts. Life Sci. 2008;82:210–7. https://doi.org/10.1016/j.lfs.2007.11.003
Todd JL, Kelly FL, Nagler A, Banner K, Pavlisko EN, Belperio JA, et al. Amphiregulin contributes to airway remodeling in chronic allograft dysfunction after lung transplantation. Am J Transplant. 2020;20:825–33. https://doi.org/10.1111/ajt.15667
Krishnan S, Goble JM, Frederick LA, James CD, Uhm JH, Kaufmann SH. et al. Inhibitory effect of pirfenidone on glioblastoma cell lines: implications for treatment of neurofibromatosis. J Appl Res. 2007;7:11
Greenland JR, Sun H, Calabrese D, Chong T, Singer JP, Kukreja J, et al. HLA mismatching favoring host-versus-graft NK cell activity Via KIR3DL1 is associated with improved outcomes following lung transplantation. Am J Transplant. 2017;17:2192–9. https://doi.org/10.1111/ajt.14295
Fang X, Zhu X, Tang B, Song K, Yao W, Wan X, et al. Donor KIR3DL1/receptor HLA-Bw4-80I combination reduces acute leukemia relapse after umbilical cord blood transplantation without in vitro T-cell depletion. Mediterr J Hematol Infect Dis. 2021;13:e2021005 https://doi.org/10.4084/MJHID.2021.005
Kasper M, Lackie P, Haase M, Schuh D, Muller M. Immunolocalization of cathepsin D in pneumocytes of normal human lung and in pulmonary fibrosis. Virchows Arch. 1996;428:207–15. https://doi.org/10.1007/BF00196692
Acknowledgements
We would like to thank all patients for their willingness to participate in the study. We would also like to thank Dr. Sally Glaser for her critical read of the manuscript and Mr. Guy Livneh for his advocacy for patients with BOS and support of the Stanford Lung GVHD clinic.
Funding
JH is funded by K08HL122528-01A1-NIH/NHLBI, and R01HL157414-01-NIH/NHLBI. GSC is funded by R01HL161037-01-NIH/NHLBI, P30 CA015704-NIH/NCI and a V Foundation grant. Genentech provided drug support for the trial. The Blood and Marrow Transplant database is funded by P01 CA049605-NIH, and P01 HL075462-NIH.
Author information
Authors and Affiliations
Contributions
JH conceived and designed the study, he had full access to all the data and is the guarantor for the integrity of the data and its analysis. EIM, HS, CO’D, WC, CO, PC, TB, KM, IT, LJ, and JH collected the data. EIM, HS, CO’D, PC, IT, GSC, and JH did the data analysis and interpretation. EIM, HS, CO’D, GSC, and JH drafted the manuscript. HS, CO’D, IT, KM, PC, GSC, and JH verified the data. All authors contributed to revisions of the manuscript. The final version of the manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
JH was supported by the National Institutes of Health (NIH) during the period that the study was conducted. GSC reports grants from NIH and the V Foundation during the study period. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Notation of prior abstract presentation: American Thoracic Society 2020.
Supplementary information
Rights and permissions
About this article
Cite this article
Matthaiou, E.I., Sharifi, H., O’Donnell, C. et al. The safety and tolerability of pirfenidone for bronchiolitis obliterans syndrome after hematopoietic cell transplant (STOP-BOS) trial. Bone Marrow Transplant 57, 1319–1326 (2022). https://doi.org/10.1038/s41409-022-01716-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01716-4