Abstract
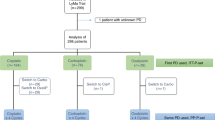
Cytarabine-based immuno-chemotherapy followed by autologous stem cell transplantation (ASCT) consolidation is standard of care for fit patients with Mantle Cell Lymphoma (MCL). BEAM (Carmustine, Etoposide, Aracytine, Melphalan) is among the most frequently used conditioning regimen. Studies comparing BEAM with Bendamustine-EAM (BeEAM) have suggested that patients treated with BeEAM have a better progression-free survival (PFS). We performed a cross-study analysis to better evaluate BeEAM. Thirty-five patients from a retrospective study who received R-DHAP/BeEAM were compared to 245 patients from the LyMa trial (NCT00921414) who all received R-DHAP followed by R-BEAM. PFS and Overall Survival (OS) were estimated using Kaplan–Meier methods. At 2 years there was no difference between R-BEAM and BeEAM in either PFS (84.9% versus 87.9%; p = 0.95) or OS (91.8% versus 94.2%; p = 0.30). Analyses were repeated on a propensity score to reduce biases. Each patient from the BeEAM cohort (n = 30) was matched to three patients from the R-BEAM cohort (n = 90) for age, sex, MIPI score, pre-transplant status disease and rituximab maintenance (RM). PFS and OS at 2 years remained similar between R-BEAM and BeEAM with more renal toxicity in BeEAM group. MCL patients who received R-DHAP induction before ASCT have similar outcome after R-BEAM or BeEAM conditioning regimen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Smedby KE, Hjalgrim H. Epidemiology and etiology of mantle cell lymphoma and other non-Hodgkin lymphoma subtypes. Semin Cancer Biol. 2011;21:293–8.
Wang Y, Ma S. Risk factors for etiology and prognosis of mantle cell lymphoma. Expert Rev Hematol. 2014;7:233–43.
Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, Thieblemont C, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388:565–75.
Le Gouill S, Thieblemont C, Oberic L, Moreau A, Bouabdallah K, Dartigeas C, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250–60.
Visani G, Malerba L, Stefani PM, Capria S, Galieni P, Gaudio F, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood. 2011;118:3419–25.
Chantepie SP, Garciaz S, Tchernonog E, Peyrade F, Larcher M-V, Diouf M, et al. Bendamustine-based conditioning prior to autologous stem cell transplantation (ASCT): results of a French multicenter study of 474 patients from LYmphoma Study Association (LYSA) centers. Am J Hematol. 2018;93:729–35.
Ghesquières H, Dalban C, Nicolas-Virelizier E, Jardin F, Le Bras F, Le, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) prior to autologous stem cell transplant for chemosensitive relapses in patients with follicular lymphoma: a prospective multicentre phase II study in Lymphoma Study Association centres†. Br J Haematol. 2021;192:e94–8.
Hueso T, Gastinne T, Garciaz S, Tchernonog E, Delette C, Casasnovas R-O, et al. Bendamustine-EAM versus BEAM regimen in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in the frontline setting: a multicenter retrospective study from Lymphoma Study Association (LYSA) centers. Bone Marrow Transpl. 2020;55:1076–84.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17:1244.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with Ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16.
Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739–45.
Garciaz S, Coso D, Schiano de Collela J-M, Broussais F, Stoppa A-M, Aurran T, et al. Bendamustine-based conditioning for non-Hodgkin lymphoma autologous transplantation: an increasing risk of renal toxicity. Bone Marrow Transpl. 2016;51:319–21.
Acknowledgements
SLG and DCT designed the research study. TH, TG, CT, LO, KB, SG, ET, CDa, VR, ROC, RH, CDe, SM, LMF, RG, GD and SLG provided data. PF performed the statistical analyses. DC, PF and SLG analyzed the data and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Costes-Tertrais, D., Hueso, T., Gastinne, T. et al. Bendamustine-EAM versus R-BEAM after high-dose cytarabine-based induction in newly diagnosed patients with mantle cell lymphoma, a LYSA retrospective study. Bone Marrow Transplant 57, 627–632 (2022). https://doi.org/10.1038/s41409-022-01596-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01596-8