Abstract
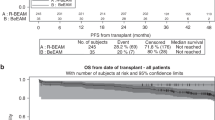
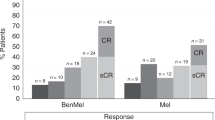
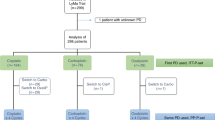
The combination of carmustine, etoposide, cytarabine, and melphalan (BEAM) as conditioning regimen prior to autologous stem-cell transplantation (ASCT) remains the standard of care for patients with mantle cell lymphoma (MCL) who are eligible for transplantation. The replacement of carmustine with bendamustine (BeEAM) was described as a promising alternative in non-Hodgkin lymphoma. The aim of this retrospective study was to compare the BeEAM with the BEAM regimen in MCL patients in the frontline setting. Sixty and 108 patients were included in the BeEAM and the BEAM groups, respectively. At 3 years, progression-free survival (PFS) was significantly higher in the BeEAM than in the BEAM group (84% [73–96] vs. 63% [51–79], p = 0.03). The overall survival was not statistically different between the two groups (p = 0.2). In multivariable analysis, BeEAM regimen remained associated with higher PFS (HR = 0.377, 95% CI, 0.146–0.970; p = 0.043). Subgroup analyses in patients treated with prior rituximab–aracytine induction alone showed that BeEAM improved the PFS compared with BEAM regimen (p = 0.04). Despite the high rate of acute renal failure KDIGO III (32%), treatment-related mortality was not increased with the BeEAM regimen. A prospective randomized trial will be necessary to confirm the beneficial effect of the BeEAM regimen in MCL patients undergoing ASCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hoster E, Dreyling M, Klapper W, Gisselbrecht C, Van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–66.
Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs porgession-free survival in mantle-cell lymphoma: results of prospective randomized trial of the European MCL. Blood. 2005;105:2677–84.
Delarue R, Haioun C, Ribrag V, Brice P, Delmer A, Tilly H, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121:48–53.
Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, Thieblemont C, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lympho. Lancet. 2016;388:565–75.
Czuczman MS, Goy A, Lamonica D, Graf DA, Munteanu MC, Van der Jagt RH. Phase II study of bendamustine combined with rituximab in relapsed / refractory mantle cell lymphoma: efficacy, tolerability, and safety findings. Ann Hematol. 2015;94:2025–32.
Visco C, Finotto S, Zambello R, Paolini R, Menin A, Zanotti R, et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol. 2013;31:1442–9.
Rummel MJ, Niederle N, Maschmeyer G, Banat GA, Von Grünhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–10.
Flinn IW, Van DerJagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–52.
Visco C, Chiappella A, Nassi L, Patti C, Ferrero S, Barbero D, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol. 2017;4:e15–23.
Flinn IW, Van DerJagt R, Kahl B, Wood P, Hawkins T, Macdonald D. First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: Results of the bright 5-year follow-up study. J Clin Oncol. 2019;37:984–92.
Anderson C, Goldstone A, Souhami RL, Linch DC, Harper PG, McLennan KA, et al. Very high dose chemotherapy with autologous bone marrow rescue in adult patients with resistant relapsed lymphoma. Cancer Chemother Pharm. 1986;16:170–5.
Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin’s lymphoma. J Clin Oncol. 1995;13:588–95.
Damaj G, Cornillon J, Bouabdallah K, Gressin R, Vigouroux S, Gastinne T, et al. Carmustine replacement in intensive chemotherapy preceding reinjection of autologous HSCs in Hodgkin and non-Hodgkin lymphoma: a review. Bone Marrow Transplant. 2017;52:941–9.
Roué G, Lopez-Guerra M, Milpied P, Pérez-Galan P, Villamor N, Montserrat E, et al. Bendamustine is effective in p53-deficient B-cell neoplasms and requires oxidative stress and caspase-independent signaling. Clin Cancer Res. 2008;14:6907–16.
Visco C, Castegnaro S, Chieregato K, Bernardi M, Albiero E, Zanon C, et al. The cytotoxic effects of bendamustine in combination with cytarabine in mantle cell lymphoma cell lines. Blood Cells, Mol Dis. 2012;48:68–75.
Visani G, Malerba L, Stefani PM, Capria S, Galieni P, Gaudio F, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood. 2011;118:3419–26.
Visani G, Stefani PM, Capria S, Malerba L, Galieni P, Gaudio F, et al. Bendamustine, etoposide, cytarabine, melphalan, and autologous stem cell rescue produce à 72% 3-years PFS in resistant lymphoma. Blood. 2014;124:3029–31.
Touzeau C, Leux C, Bouabdallah R, Roussel M, Delarue R, Bouabdallah K, et al. Autologous stem cell transplantation in mantle cell lymphoma: a report from the SFGM-TC. Ann Hematol. 2014;93:233–42.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244.
Chantepie SP, Garciaz S, Tchernonog E, Peyrade F, Larcher MV, Diouf M, et al. Bendamustine-based conditioning prior to autologous stem cell transplantation (ASCT): results of a French multicenter study of 474 patients from LYmphoma Study Association (LYSA) centers. Am J Hematol. 2018;93:729–35.
Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transpl. 2007;40:381–7.
Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transpl. 2010;45:1388–95.
Le Gouill S, Thieblemont C, Oberic L, Moreau A, Bouabdallah K, Dartigeas C, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N. Engl J Med. 2017;377:1250–60.
Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Trneny M, Geisler CH, et al. Treatment of older patients with mantle-cell lymphoma. N. Engl J Med. 2012;367:520–31.
Rummel MJ, Knauf W, Goerner M, Soeling U, Lange E, Hertenstein B, et al. Two years rituximab maintenance vs. observation after first-line treatment with bendamustine plus rituximab (B-R) in patients with mantle cell lymphoma: First results of a prospective, randomized, multicenter phase II study (a subgroup study of the StiL NHL7-2008 MAINTAIN Trial). J. Clin. Oncol. 2016;34:(15_suppl)7503.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl J Med. 2013;369:507–16.
Redondo AM, Valcárcel D, González-Rodríguez AP, Suárez-Lledó M, Bello JL, Canales M, et al. Bendamustine as part of conditioning of autologous stem cell transplantation in patients with aggressive lymphoma: a phase 2 study from the GELTAMO group. Br J Haematol. 2019;184:797–807.
Gilli S, Novak U, Taleghani BM, Baerlocher GM, Leibundgut K, Banz Y, et al. BeEAM conditioning with bendamustine-replacing BCNU before autologous transplantation is safe and effective in lymphoma patients. Ann Hematol. 2017;96:421–9.
Noesslinger T, Panny M, Simanek R, Moestl M, Boehm A, Menschel E, et al. High-dose Bendamustine-EAM followed by autologous stem cell rescue results in long-term remission rates in lymphoma patients, without renal toxicity. Eur J Haematol. 2018;101:326–31.
Prediletto I, Farag SA, Bacher U, Jeker B, Mansouri Taleghani B, Brégy R, et al. High incidence of reversible renal toxicity of dose-intensified bendamustine-based high-dose chemotherapy in lymphoma and myeloma patients. Bone Marrow Transplant. 2019;54:1923–25.
Saleh K, Danu A, Koscielny S, Legoupil C, Pilorge S, Castilla-llorente C, et al. A retrospective, matched paired analysis comparing bendamustine containing BeEAM versus BEAM conditioning regimen: results from a single center experience. Leuk Lymphoma. 2018;59:2580–7.
Jo JC, Kang BW, Jang G, Sym SJ, Lee SS, Koo JE, et al. BEAC or BEAM high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin’ s lymphoma patients: comparative analysis of efficacy and toxicity. Ann Hematol. 2008;87:43–8.
Olivieri J, Mosna F, Pelosini M, Fama A, Rattotti S, Giannoccaro M, et al. A comparison of the conditioning regimens BEAM and FEAM for autologous hematopoietic stem cell transplantation in lymphoma: an observational study on 1038 patients from fondazione Italiana Linfomi. Biol Blood Marrow Transplant. 2018;24:1814–22.
Author information
Authors and Affiliations
Contributions
TH and GD designed and wrote the paper. TH and RM performed all statistical analyses. All authors reviewed and approved the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hueso, T., Gastinne, T., Garciaz, S. et al. Bendamustine-EAM versus BEAM regimen in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in the frontline setting: a multicenter retrospective study from Lymphoma Study Association (LYSA) centers. Bone Marrow Transplant 55, 1076–1084 (2020). https://doi.org/10.1038/s41409-020-0783-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0783-y
This article is cited by
-
Comparison of MEAM, MCEC and LEED high-dose chemotherapy followed by autologous stem cell transplantation in relapsed/refractory diffuse large B-cell lymphoma: data from the Japan Society for Hematopoietic and Cellular Therapy Registry
Bone Marrow Transplantation (2024)
-
Stem cell transplant for mantle cell lymphoma in Taiwan
Scientific Reports (2022)
-
Bendamustine-EAM versus R-BEAM after high-dose cytarabine-based induction in newly diagnosed patients with mantle cell lymphoma, a LYSA retrospective study
Bone Marrow Transplantation (2022)
-
Antineoplastics
Reactions Weekly (2020)