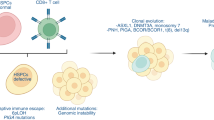
Abstract
This review highlights literature pertinent to chimerism analysis in the context of hematopoietic cell transplantation (HCT). We also conducted a survey of testing practices of program members of CIBMTR worldwide. Questions included testing methods, time points, specimen type, cell lineage tested and testing indications. Recent literature suggests that detection of low level mixed chimerism has a clinical utility in predicting relapse. There is also increasing recognition of HLA loss relapse to potentially guide rescue decisions in cases of relapse. These developments coincide with wider access to high sensitivity next generation sequencing (NGS) in clinical laboratories. Our survey revealed a heterogeneity in practices as well as in findings and conclusions of published studies. Although the most commonly used method is STR, studies support more sensitive methods such as NGS, especially for predicting relapse. There is no conclusive evidence to support testing chimerism in BM over PB, particularly when using a high sensitivity testing method. Periodic monitoring of chimerism especially in diagnoses with a high risk of relapse is advantageous. Lineage specific chimerism is more sensitive than whole blood in predicting impending relapse. Further studies that critically assess how to utilize chimerism testing results will inform evidence based clinical management decisions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Van Deerlin VM, Leonard DG. Bone marrow engraftment analysis after allogeneic bone marrow transplantation. Clin Lab Med. 2000;20:197–225.
Qin XY, Li GX, Qin YZ, Wang Y, Wang FR, Liu DH, et al. Quantitative chimerism: an independent acute leukemia prognosis indicator following allogeneic hematopoietic SCT. Bone Marrow Transpl. 2014;49:1269–77.
Bacher U, Haferlach T, Fehse B, Schnittger S, Kroger N. Minimal residual disease diagnostics and chimerism in the post-transplant period in acute myeloid leukemia. ScientificWorldJournal 2011;11:310–9.
Ahci M, Stempelmann K, Buttkereit U, Crivello P, Trilling M, Heinold A, et al. Clinical utility of quantitative PCR for chimerism and engraftment monitoring after allogeneic stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2017;23:1658–68.
Unnikrishnan A, Meacham AM, Goldstein SS, Ta M, Leather HL, Cogle CR, et al. CD34+ chimerism analysis for minimal residual disease monitoring after allogeneic hematopoietic cell transplantation. Leuk Res. 2018;74:110–2.
Khan F, Agarwal A, Agrawal S. Significance of chimerism in hematopoietic stem cell transplantation: new variations on an old theme. Bone Marrow Transpl. 2004;34:1–12.
Pedini P, Cherouat N, Basire A, Simon S, Budon L, Pourtein M, et al. Evaluation of Next-Generation Sequencing and Crystal Digital PCR for Chimerism Monitoring of Post-Allogeneic Hematopoietic Stem Cell Transplantation. Transplant Cell Ther. 2021;27:89.e1–e10.
Ahci M, Toffalori C, Bouwmans E, Crivello P, Brambati C, Pultrone C, et al. A new tool for rapid and reliable diagnosis of HLA loss relapses after HSCT. Blood 2017;130:1270–3.
Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. The. N. Engl J Med. 2009;361:478–88.
Antin JH, Childs R, Filipovich AH, Giralt S, Mackinnon S, Spitzer T, et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2001;7:473–85.
Blouin AG, Ye F, Williams J, Askar M. A practical guide to chimerism analysis: Review of the literature and testing practices worldwide. Hum Immunol. 2021;82:838–49.
Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C, et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood 2002;99:4618–25.
Gineikiene E, Stoskus M, Griskevicius L. Single nucleotide polymorphism-based system improves the applicability of quantitative PCR for chimerism monitoring. J Mol Diagn. 2009;11:66–74.
Pettersson L, Vezzi F, Vonlanthen S, Alwegren K, Hedrum A, Hauzenberger D. Development and performance of a next generation sequencing (NGS) assay for monitoring of mixed chimerism. Clin Chim Acta. 2020;512:40–8.
Bader P, Niethammer D, Willasch A, Kreyenberg H, Klingebiel T. How and when should we monitor chimerism after allogeneic stem cell transplantation? Bone Marrow Transpl. 2005;35:107–19.
Stahl T, Rothe C, Böhme MU, Kohl A, Kröger N, Fehse B. Digital PCR Panel for Sensitive Hematopoietic Chimerism Quantification after Allogeneic Stem Cell Transplantation. Int J Mol Sci. 2016;17:1515.
Horn B, Soni S, Khan S, Petrovic A, Breslin N, Cowan M, et al. Feasibility study of preemptive withdrawal of immunosuppression based on chimerism testing in children undergoing myeloablative allogeneic transplantation for hematologic malignancies. Bone Marrow Transpl. 2009;43:469–76.
Ladetto M, Bruggemann M, Monitillo L, Ferrero S, Pepin F, Drandi D, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia 2014;28:1299–307.
Pettersson L, Vezzi F, Vonlanthen S, Alwegren K, Hedrum A, Hauzenberger D. Development and performance of a next generation sequencing (NGS) assay for monitoring of mixed chimerism. Clin Chim Acta. 2021;512:40–8.
George D, Czech J, John B, Yu M, Jennings LJ. Detection and quantification of chimerism by droplet digital PCR. Chimerism 2013;4:102–8.
Waterhouse M, Pfeifer D, Follo M, Duyster J, Schafer H, Bertz H, et al. Early mixed hematopoietic chimerism detection by digital droplet PCR in patients undergoing gender-mismatched hematopoietic stem cell transplantation. Clin Chem Lab Med. 2017;55:1115–21.
Andrikovics H, Őrfi Z, Meggyesi N, Bors A, Varga L, Kövy P, et al. Current trends in applications of circulatory microchimerism detection in transplantation. Int J Mol Sci. 2019;20:4450–68.
Pedini P, Kouba N, Riquier M, Simon S, Basire A, Fina F, et al. Droplet digital PCR: a new technology for detection and quantification of chimerism after allogenic hematopoietic stem cell transplantation. Biomed J of Scientific & Technical Res. 2019;13:10065–8.
Kliman D, Castellano-Gonzalez G, Withers B, Street J, Tegg E, Mirochnik O, et al. Ultra-sensitive droplet digital PCR for the assessment of microchimerism in cellular therapies. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2018;24:1069–78.
Mika T, Baraniskin A, Ladigan S, Wulf G, Dierks S, Haase D, et al. Digital droplet PCR-based chimerism analysis for monitoring of hematopoietic engraftment after allogeneic stem cell transplantation. Int J Lab Hematol. 2019;41:615–21.
Valero-Garcia J, González-Espinosa MDC, Barrios M, Carmona-Antoñanzas G, García-Planells J, Ruiz-Lafora C, et al. Earlier relapse detection after allogeneic haematopoietic stem cell transplantation by chimerism assays: digital PCR versus quantitative real-time PCR of insertion/deletion polymorphisms. PLoS One. 2019;14:e0212708.
Stahl T, Bohme MU, Kroger N, Fehse B. Digital PCR to assess hematopoietic chimerism after allogeneic stem cell transplantation. Exp Hematol. 2015;43:462–8.e1.
Duke JL, Lind C, Mackiewicz K, Ferriola D, Papazoglou A, Gasiewski A, et al. Determining performance characteristics of an NGS-based HLA typing method for clinical applications. HLA. 2016;87:141–52.
Smith AG, Pereira S, Jaramillo A, Stoll ST, Khan FM, Berka N, et al. Comparison of sequence-specific oligonucleotide probe vs next generation sequencing for HLA-A, B, C, DRB1, DRB3/B4/B5, DQA1, DQB1, DPA1, and DPB1 typing: Toward single-pass high-resolution HLA typing in support of solid organ and hematopoietic cell transplant programs. Hla. 2019;94:296–306.
Haugaard AK, Kofoed J, Masmas TN, Madsen HO, Marquart HV, Heilmann C, et al. Is microchimerism a sign of imminent disease recurrence after allogeneic hematopoietic stem cell transplantation? A systematic review of the literature. Blood Rev. 2020;44:100673.
Lassaletta A, Ramirez M, Montero JM, Gonzalez-Vicent M, Balas A, Madero L, et al. Full donor chimerism by day 30 after allogeneic peripheral blood progenitor cell transplantation is associated with a low risk of relapse in pediatric patients with hematological malignancies. Leukemia 2005;19:504–6.
Liou A, Wahlstrom JT, Dvorak CC, Horn BN. Safety of pre-emptive donor lymphocyte infusions (DLI) based on mixed chimerism (MC) in peripheral blood or bone marrow subsets in children undergoing hematopoietic stem cell transplant (HSCT) for hematologic malignancies. Bone Marrow Transpl. 2017;52:1057–9.
Rettinger E, Merker M, Salzmann-Manrique E, Kreyenberg H, Krenn T, Durken M, et al. Pre-emptive immunotherapy for clearance of molecular disease in childhood acute lymphoblastic leukemia after transplantation. Biol Blood Marrow Transpl. 2017;23:87–95.
Chen CT, Gau JP, Liu JH, Chiou TJ, Hsiao LT, Liu YC. Early achievement of full donor chimerism after allogeneic hematopoietic stem cell transplantation predicts lower relapse risk in patients with acute lymphoblastic leukemia. J Chin Med Assoc. 2018;81:1038–43.
Rauwerdink CA, Tsongalis GJ, Tosteson TD, Hill JM, Meehan KR. The practical application of chimerism analyses in allogeneic stem cell transplant recipients: blood chimerism is equivalent to marrow chimerism. Exp Mol Pathol. 2012;93:339–44.
Lejman M, Zawitkowska J, Zaucha-Prazmo A, Cienkusz M, Mroczkowska A, Kowalczyk J, et al. Influence of mixed chimerism on outcome in children with anaemia after haematopoietic stem cell transplantation. Vivo. 2019;33:2051–7.
Doney K, Loken M, Bryant E, Smith A, Appelbaum F. Lack of utility of chimerism studies obtained 2–3 months after myeloablative hematopoietic cell transplantation for ALL. Bone Marrow Transpl. 2008;42:271–4.
Mossallam GI, Kamel AM, Storer B, Martin PJ. Prognostic utility of routine chimerism testing at 2 to 6 months after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2009;15:352–9.
Dillon LW, Gui G, Logan BR, Fei M, Ghannam J, Li Y, et al. Impact of conditioning intensity and genomics on relapse after allogeneic transplantation for patients with myelodysplastic syndrome. JCO Precis Oncol. 2021;5:265–73.
Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–9.
Atsuta Y, Hirakawa A, Nakasone H, Kurosawa S, Oshima K, Sakai R, et al. Late mortality and causes of death among long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transpl. 2016;22:1702–9.
Solh MM, Bashey A, Solomon SR, Morris LE, Zhang X, Brown S, et al. Long term survival among patients who are disease free at 1-year post allogeneic hematopoietic cell transplantation: a single center analysis of 389 consecutive patients. Bone Marrow Transpl. 2018;53:576–83.
Bhatia S. Cause-specific late mortality after allogeneic stem cell transplantation. Hematol Am Soc Hematol Educ Program. 2019;2019:626–9.
Ramirez P, Wagner JE, DeFor TE, Blazar BR, Verneris MR, Miller JS, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transpl. 2012;47:799–803.
Newell LF, Milano F, Nicoud IB, Pereira S, Gooley TA, Heimfeld S, et al. Early CD3 peripheral blood chimerism predicts the long-term engrafting unit following myeloablative double-cord blood transplantation. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2012;18:1243–9.
Avery S, Voss MH, Gonzales AM, Lubin M, Castro-Malaspina H, Giralt S, et al. Importance of day 21 BM chimerism in sustained neutrophil engraftment following double-unit cord blood transplantation. Bone Marrow Transpl. 2012;47:1056–60.
Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front in Immunol. 2016;7:507.
Llaurador G, Nicoletti E, Prockop SE, Hsu S, Fuller K, Mauguen, A, et al. Donor-Host Lineage-Specific Chimerism Monitoring and Analysis in Pediatric Patients Following Allogeneic Stem Cell Transplantation: Influence of Pretransplantation Variables and Correlation with Post-Transplantation Outcomes. Transplant Cell Ther 2021;27:780.e1–e14.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22:1696–705.
Baron F, Baker JE, Storb R, Gooley TA, Sandmaier BM, Maris MB, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 2004;104:2254–62.
Bornhäuser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG, et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica 2009;94:1613–7.
Breuer S, Preuner S, Fritsch G, Daxberger H, Koenig M, Poetschger U, et al. Early recipient chimerism testing in the T- and NK-cell lineages for risk assessment of graft rejection in pediatric patients undergoing allogeneic stem cell transplantation. Leukemia 2012;26:509–19.
Lee HC, Saliba RM, Rondon G, Chen J, Charafeddine Y, Medeiros LJ, et al. Mixed T lymphocyte chimerism after allogeneic hematopoietic transplantation is predictive for relapse of acute myeloid leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2015;21:1948–54.
Lion T, Daxberger H, Dubovsky J, Filipcik P, Fritsch G, Printz D, et al. Analysis of chimerism within specific leukocyte subsets for detection of residual or recurrent leukemia in pediatric patients after allogeneic stem cell transplantation. Leukemia 2001;15:307–10.
Matthes-Martin S, Lion T, Haas OA, Frommlet F, Daxberger H, Konig M, et al. Lineage-specific chimaerism after stem cell transplantation in children following reduced intensity conditioning: potential predictive value of NK cell chimaerism for late graft rejection. Leukemia 2003;17:1934–42.
Miura Y, Tanaka J, Toubai T, Tsutsumi Y, Kato N, Hirate D, et al. Analysis of donor-type chimerism in lineage-specific cell populations after allogeneic myeloablative and non-myeloablative stem cell transplantation. Bone Marrow Transpl. 2006;37:837–43.
Thiede C, Bornhauser M, Oelschlagel U, Brendel C, Leo R, Daxberger H, et al. Sequential monitoring of chimerism and detection of minimal residual disease after allogeneic blood stem cell transplantation (BSCT) using multiplex PCR amplification of short tandem repeat-markers. Leukemia 2001;15:293–302.
Preuner S, Peters C, Potschger U, Daxberger H, Fritsch G, Geyeregger R, et al. Risk assessment of relapse by lineage-specific monitoring of chimerism in children undergoing allogeneic stem cell transplantation for acute lymphoblastic leukemia. Haematologica 2016;101:741–6.
van Besien K, Koshy N, Gergis U, Mayer S, Cushing M, Rennert H, et al. Cord blood chimerism and relapse after haplo-cord transplantation. Leuk Lymphoma. 2017;58:288–97.
Bernal T, Diez-Campelo M, Godoy V, Rojas S, Colado E, Alcoceba M, et al. Role of minimal residual disease and chimerism after reduced-intensity and myeloablative allo-transplantation in acute myeloid leukemia and high-risk myelodysplastic syndrome. Leuk Res. 2014;38:551–6.
Nikolousis E, Robinson S, Nagra S, Brookes C, Kinsella F, Tauro S, et al. Post-transplant T cell chimerism predicts graft versus host disease but not disease relapse in patients undergoing an alemtuzumab based reduced intensity conditioned allogeneic transplant. Leuk Res. 2013;37:561–5.
Deeg HJ, Salit RB, Monahan T, Schoch G, McFarland C, Scott BL, et al. Early mixed lymphoid donor/host chimerism is associated with improved transplant outcome in patients with primary or secondary myelofibrosis. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2020;26:2197–203.
Pichler H, Fritsch G, Konig M, Daxberger H, Glogova E, Potschger U, et al. Peripheral blood late mixed chimerism in leucocyte subpopulations following allogeneic stem cell transplantation for childhood malignancies: does it matter? Br J Haematol. 2016;173:905–17.
Serrano J, Roman J, Sanchez J, Jimenez A, Castillejo JA, Herrera C, et al. Molecular analysis of lineage-specific chimerism and minimal residual disease by RT-PCR of p210(BCR-ABL) and p190(BCR-ABL) after allogeneic bone marrow transplantation for chronic myeloid leukemia: increasing mixed myeloid chimerism and p190(BCR-ABL) detection precede cytogenetic relapse. Blood 2000;95:2659–65.
Srour SA, Olson A, Ciurea SO, Desai P, Bashir Q, Oran B, et al. Mixed myeloid chimerism and relapse of myelofibrosis after allogeneic stem cell transplantation. Haematologica 2021;106:1988–90.
Mountjoy L, Palmer J, Kunze KL, Khera N, Sproat LZ, Leis JF, et al. Does early chimerism testing predict outcomes after allogeneic hematopoietic stem cell transplantation? Leuk Lymphoma. 2021;62:252–4.
Jiang Y, Wan L, Qin Y, Wang X, Yan S, Xie K, et al. Donor chimerism of B cells and nature killer cells provides useful information to predict hematologic relapse following allogeneic hematopoietic stem cell transplantation. PLoS One. 2015;10:e0133671.
Yang YN, Wang XR, Qin YW, Wan LP, Jiang Y, Wang C. Is there a role for B lymphocyte chimerism in the monitoring of B-acute lymphoblastic leukemia patients receiving allogeneic stem cell transplantation? Chronic Dis Transl Med. 2015;1:48–54.
Zetterquist H, Mattsson J, Uzunel M, Näsman-Björk I, Svenberg P, Tammik L, et al. Mixed chimerism in the B cell lineage is a rapid and sensitive indicator of minimal residual disease in bone marrow transplant recipients with pre-B cell acute lymphoblastic leukemia. Bone Marrow Transpl. 2000;25:843–51.
Zeiser R, Spyridonidis A, Wasch R, Ihorst G, Grullich C, Bertz H, et al. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation. Leukemia 2005;19:814–21.
Rosenow F, Berkemeier A, Krug U, Muller-Tidow C, Gerss J, Silling G, et al. CD34(+) lineage specific donor cell chimerism for the diagnosis and treatment of impending relapse of AML or myelodysplastic syndrome after allo-SCT. Bone Marrow Transpl. 2013;48:1070–6.
Qin XY, Li GX, Qin YZ, Wang Y, Wang FR, Liu DH, et al. Quantitative chimerism kinetics in relapsed leukemia patients after allogeneic hematopoietic stem cell transplantation. Chin Med J (Engl). 2012;125:1952–9.
Waterhouse M, Pfeifer D, Duque-Afonso J, Follo M, Duyster J, Depner M, et al. Droplet digital PCR for the simultaneous analysis of minimal residual disease and hematopoietic chimerism after allogeneic cell transplantation. Clin Chem Lab Med. 2019;57:641–7.
Bach C, Steffen M, Roesler W, Winkler J, Mackensen A, Stachel KD, et al. Systematic comparison of donor chimerism in peripheral blood and bone marrow after hematopoietic stem cell transplantation. Blood Cancer J. 2017;7:e566.
Navarro-Bailon A, Carbonell D, Escudero A, Chicano M, Muniz P, Suarez-Gonzalez J, et al. Short tandem repeats (STRs) as biomarkers for the quantitative follow-up of chimerism after stem cell transplantation: methodological considerations and clinical application. Genes (Basel). 2020;11:993.
Gambacorta V, Parolini R, Xue E, Greco R, Bouwmans EE, Toffalori C, et al. Quantitative PCR-based chimerism in bone marrow or peripheral blood to predict acute myeloid leukemia relapse in high-risk patients: results from the KIM-PB prospective study. Haematologica. 2020;Online ahead of print.
Bartsch K, Al-Ali H, Reinhardt A, Franke C, Hudecek M, Kamprad M, et al. Mesenchymal stem cells remain host-derived independent of the source of the stem-cell graft and conditioning regimen used. Transplantation 2009;87:217–21.
Karasawa M, Yamane A, Mitsui T, Irisawa H, Sakura T, Matsushima T, et al. Long-term persistence of host cells detected by X-chromosome gene-based assay in patients undergoing gender-mismatched hematopoietic stem cell transplantation. Am J Hematol. 2005;80:101–5.
Buchta C, Nedorost N, Regele H, Egerbacher M, Körmöczi G, Höcker P, et al. Skin plugs in phlebotomy puncture for blood donation. Wien Klin Wochenschr. 2005;117:141–4.
Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B, et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood 2011;118:5681–8.
Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C, et al. Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood 1994;84:3948–55.
Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood 1998;91:1083–90.
Bader P, Beck J, Frey A, Schlegel PG, Hebarth H, Handgretinger R, et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transpl. 1998;21:487–95.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Kremens B, Dilloo D, et al. Increasing mixed chimerism defines a high-risk group of childhood acute myelogenous leukemia patients after allogeneic stem cell transplantation where pre-emptive immunotherapy may be effective. Bone Marrow Transpl. 2004;33:815–21.
Barrios M, Jiménez-Velasco A, Román-Gómez J, Madrigal ME, Castillejo JA, Torres A, et al. Chimerism status is a useful predictor of relapse after allogeneic stem cell transplantation for acute leukemia. Haematologica 2003;88:801–10.
Serrano J, Román J, Herrera C, Castillejo JA, Navarro JA, Reina ML, et al. Increasing mixed haematopoietic chimaerism after BMT with total depletion of CD4+ and partial depletion of CD8+ lymphocytes is associated with a higher incidence of relapse. Bone Marrow Transpl. 1999;23:475–82.
Winiarski J, Gustafsson A, Wester D, Dalianis T. Follow-up of chimerism, including T- and B-lymphocytes and granulocytes in children more than one year after allogeneic bone marrow transplantation. Pediatr Transpl. 2000;4:132–9.
Suttorp M, Schmitz N, Dreger P, Schaub J, Löffler H. Monitoring of chimerism after allogeneic bone marrow transplantation with unmanipulated marrow by use of DNA polymorphisms. Leukemia 1993;7:679–87.
Choi SJ, Lee KH, Lee JH, Kim S, Chung HJ, Lee JS, et al. Prognostic value of hematopoietic chimerism in patients with acute leukemia after allogeneic bone marrow transplantation: a prospective study. Bone Marrow Transpl. 2000;26:327–32.
Molloy K, Goulden N, Lawler M, Cornish J, Oakhill A, Pamphilon D, et al. Patterns of hematopoietic chimerism following bone marrow transplantation for childhood acute lymphoblastic leukemia from volunteer unrelated donors. Blood 1996;87:3027–31.
Jacque N, Nguyen S, Golmard JL, Uzunov M, Garnier A, Leblond V, et al. Chimerism analysis in peripheral blood using indel quantitative real-time PCR is a useful tool to predict post-transplant relapse in acute leukemia. Bone Marrow Transpl. 2015;50:259–65.
Sellmann L, Rabe K, Bünting I, Dammann E, Göhring G, Ganser A, et al. Diagnostic value of highly-sensitive chimerism analysis after allogeneic stem cell transplantation. Bone Marrow Transpl. 2018;53:1457–65.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9.
Horn B, Petrovic A, Wahlstrom J, Dvorak CC, Kong D, Hwang J, et al. Chimerism-based pre-emptive immunotherapy with fast withdrawal of immunosuppression and donor lymphocyte infusions after allogeneic stem cell transplantation for pediatric hematologic malignancies. Biol Blood Marrow Transpl. 2015;21:729–37.
Patriarca F, Sperotto A, Lorentino F, Oldani E, Mammoliti S, Isola M, et al. Donor lymphocyte infusions after allogeneic stem cell transplantation in acute leukemia: a survey from the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Front Oncol. 2020;10:572918.
Gahrton G, Iacobelli S, Garderet L, Yakoub-Agha I, Schönland S. Allogeneic transplantation in multiple myeloma-does it still have a place? J Clin Med. 2020;9:2180.
Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood 2006;107:3474–80.
Gertz MA. When to recommend allogeneic transplant in multiple myeloma. Leuk Lymphoma. 2015;56:2512–7.
Kaloyannidis P, Apostolidis J. Allogeneic stem cell transplantation in patients with high-risk multiple myeloma: utopia or continuous challenge in aiming for cure? Curr Treat Options Oncol. 2021;22:65.
Kröger N, Zagrivnaja M, Schwartz S, Badbaran A, Zabelina T, Lioznov M, et al. Kinetics of plasma-cell chimerism after allogeneic stem cell transplantation by highly sensitive real-time PCR based on sequence polymorphism and its value to quantify minimal residual disease in patients with multiple myeloma. Exp Hematol. 2006;34:688–94.
Galimberti S, Benedetti E, Morabito F, Fazzi R, Pacini S, Andreazzoli F, et al. Chimerism does not influence graft-versus-myeloma and graft-versus-host disease in reduced intensity setting. Transpl Immunol. 2005;15:173–7.
Rasche L, Röllig C, Stuhler G, Danhof S, Mielke S, Grigoleit GU, et al. Allogeneic hematopoietic cell transplantation in multiple myeloma: focus on longitudinal assessment of donor chimerism, extramedullary disease, and high-risk cytogenetic features. Biol Blood Marrow Transpl. 2016;22:1988–96.
Chhabra S, Szabo A, Glisch C, George G, Narra RK, Harrington A, et al. Relapse after allogeneic hematopoietic cell transplantation for multiple myeloma: survival outcomes and factors influencing them. Biol Blood Marrow Transpl. 2020;26:1288–97.
Villalobos IB, Takahashi Y, Akatsuka Y, Muramatsu H, Nishio N, Hama A, et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood 2010;115:3158–61.
McCurdy SR, Iglehart BS, Batista DA, Gocke CD, Ning Y, Knaus HA, et al. Loss of the mismatched human leukocyte antigen haplotype in two acute myelogenous leukemia relapses after haploidentical bone marrow transplantation with post-transplantation cyclophosphamide. Leukemia 2016;30:2102–6.
Vago L, Toffalori C, Ahci M, Lange V, Lang K, Todaro S, et al. Incidence of HLA loss in a global multicentric cohort of post-transplantation relapses: results from the hlaloss collaborative study. Blood 2018;132:818.
Toffalori C, Cavattoni I, Deola S, Mastaglio S, Giglio F, Mazzi B, et al. Genomic loss of patient-specific HLA in acute myeloid leukemia relapse after well-matched unrelated donor HSCT. Blood 2012;119:4813–5.
Waterhouse M, Pfeifer D, Pantic M, Emmerich F, Bertz H, Finke J. Genome-wide profiling in AML patients relapsing after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2011;17:1450–9.e1.
Dehn J, Spellman S, Hurley CK, Shaw BE, Barker JN, Burns LJ, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood 2019;134:924–34.
Crucitti L, Crocchiolo R, Toffalori C, Mazzi B, Greco R, Signori A, et al. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation. Leukemia 2015;29:1143–52.
Vago L, Ciceri F. Choosing the alternative. Biol Blood Marrow Transpl. 2017;23:1813–4.
Morin-Zorman S, Loiseau P, Taupin JL, Caillat-Zucman S. Donor-specific Anti-HLA antibodies in allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:307.
Acknowledgements
We appreciate the support of the CIBMTR through distributing the survey to their program director members. The CIBMTR is supported primarily by Public Health Service grant/cooperative agreement U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID).
Author information
Authors and Affiliations
Contributions
MA conceived the idea; MA and AGB analyzed the survey results. MA and AGB searched and analyzed the literature and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
MA has received compensation as a member of the scientific advisory board for CareDx, Inc. AGB declares no potential competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Blouin, A.G., Askar, M. Chimerism analysis for clinicians: a review of the literature and worldwide practices. Bone Marrow Transplant 57, 347–359 (2022). https://doi.org/10.1038/s41409-022-01579-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01579-9
This article is cited by
-
Can novel methods replace the gold standard chimerism method after allogeneic hematopoietic stem cell transplantation?
Annals of Hematology (2024)
-
DNA methylation of hematopoietic stem/progenitor cells from donor peripheral blood to patient bone marrow: implications for allogeneic hematopoietic stem cell transplantation
Clinical and Experimental Medicine (2023)