Abstract
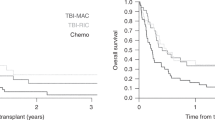
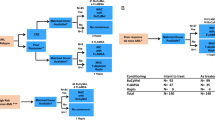
Allogeneic hematopoietic cell transplantation (allo-HCT) is the only curative treatment for patients with myeloid/lymphoid neoplasm (MLN) with FGFR1 rearrangement, but data on overall results are limited. We report on the largest series of patients (n = 22) with FGFR1-rearranged MLN undergoing allo-HCT. Distribution according to cytogenetic subtype was: t(8;13) in 11 cases, t(8;22) in 7 cases, t(6;8) in 2 cases, and other (n = 2). Over a third of patients displayed a chronic myeloproliferative (MPN) phenotype, another third showed MPN features with concomitant lymphoma or acute leukemia, and the remaining ones presented as acute leukemia. After a median follow-up of 4.1 years from transplant, the estimated 5-year survival rate, progression-free survival, non-relapse mortality and relapse incidence was 74%, 63%, 14% and 23%, respectively. Causes of death were relapse/progression (n = 4), graft-versus-host disease (n = 2) and organ toxicity (n = 1). Six patients experienced disease relapse at a median of 6.1 months (range: 2.3–119.6). Two of them achieved complete remission with ponatinib or pemigatinib and were alive at 34.5 and 37 months from relapse, respectively. These data highlight the significant curative potential of allo-HCT in this aggressive disease. Maintenance with tyrosine kinase inhibitors may be a promising approach, at least in cases with detectable residual disease after transplant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
31 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41409-022-01652-3
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Patnaik MM, Gangat N, Knudson RA, Keefe JG, Hanson CA, Pardanani A, et al. Chromosome 8p11.2 translocations: prevalence, FISH analysis for FGFR1 and MYST3, and clinicopathologic correlates in a consecutive cohort of 13 cases from a single institution. Am J Hematol. 2010;85:238–42.
Strati P, Tang G, Duose DY, Mallampati S, Luthra R, Patel KP, et al. Myeloid/lymphoid neoplasms with FGFR1 rearrangement. Leuk Lymphoma. 2018;59:1672–6.
Roumiantsev S, Krause DS, Neumann CA, Dimitri CA, Asiedu F, Cross NC, et al. Distinct stem cell myeloproliferative/T lymphoma syndromes induced by ZNF198-FGFR1 and BCR-FGFR1 fusion genes from 8p11 translocations. Cancer Cell. 2004;5:287–98.
Inhorn RC, Aster JC, Roach SA, Slapak CA, Soiffer R, Tantravahi R, et al. A syndrome of lymphoblastic lymphoma, eosinophilia, and myeloid hyperplasia/malignancy associated with t(8;13)(p11;q11): description of a distinctive clinicopathologic entity. Blood. 1995;85:1881–7.
Reiter A, Sohal J, Kulkarni S, Chase A, Macdonald DH, Aguiar RC, et al. Consistent fusion of ZNF198 to the fibroblast growth factor receptor-1 in the t(8;13)(p11;q12) myeloproliferative syndrome. Blood. 1998;92:1735–42.
Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Hum Pathol. 2010;41:461–76.
Macdonald D, Aguiar RC, Mason PJ, Goldman JM, Cross NC. A new myeloproliferative disorder associated with chromosomal translocations involving 8p11: a review. Leukemia 1995;9:1628–30.
Baer C, Muehlbacher V, Kern W, Haferlach C, Haferlach T. Molecular genetic characterization of myeloid/lymphoid neoplasms associated with eosinophilia and rearrangement of PDGFRA, PDGFRB, FGFR1 or PCM1-JAK2. Haematologica. 2018;103:e348–e50.
Khodadoust MS, Luo B, Medeiros BC, Johnson RC, Ewalt MD, Schalkwyk AS, et al. Clinical activity of ponatinib in a patient with FGFR1-rearranged mixed-phenotype acute leukemia. Leukemia. 2016;30:947–50.
Kasbekar M, Nardi V, Dal Cin P, Brunner AM, Burke M, Chen YB, et al. Targeted FGFR inhibition results in a durable remission in an FGFR1-driven myeloid neoplasm with eosinophilia. Blood Adv. 2020;4:3136–40.
Verstovsek S, Subbiah V, Masarova L, Yin CC, Tang G, Manshouri T, et al. Treatment of the myeloid/lymphoid neoplasm with FGFR1 rearrangement with FGFR1 inhibitor. Ann Oncol. 2018;29:1880–2.
Verstovsek S, Vannucchi A, Rambaldi A, Gotlib J, Mead A, Hochhaus A, et al. Interim results from fight-203, a phase 2, open-label, multicenter study evaluating the efficacy and safety of pemigatinib (INCB054828) in patients with myeloid/lymphoid neoplasms with rearrangement of fibroblast growth factor receptor 1 (FGFR1). Blood. 2018;132 :(abstract 690).
Dolan M, Cioc A, Cross NC, Neglia JP, Tolar J. Favorable outcome of allogeneic hematopoietic cell transplantation for 8p11 myeloproliferative syndrome associated with BCR-FGFR1 gene fusion. Pediatr Blood Cancer. 2012;59:194–6.
Konishi Y, Kondo T, Nakao K, Asagoe K, Otsuka Y, Nishikori M, et al. Allogeneic hematopoietic stem cell transplantation for 8p11 myeloproliferative syndrome with BCR-FGFR1 gene rearrangement: a case report and literature review. Bone Marrow Transpl. 2019;54:326–9.
Katagiri S, Umezu T, Azuma K, Kobayashi C, Akahane D, Suguro T, et al. Maintenance 5-azacytidine therapy by MRD monitoring after allogeneic HSCT in myeloid/lymphoid neoplasms with FGFR1 rearrangement. Bone Marrow Transpl. 2019;54:1148–50.
Townsend W, Cross NC, Waghorn K, Somana K, Ramsay A, Thomson K, et al. Clinical evidence for a graft-versus-tumour effect following allogeneic HSCT for t(8;13) atypical myeloproliferative disorder. Bone Marrow Transpl. 2009;44:197–9.
Suzan F, Guasch G, Terre C, Garcia I, Bastie JN, Maarek O, et al. Long-term complete haematological and molecular remission after allogeneic bone marrow transplantation in a patient with a stem cell myeloproliferative disorder associated with t(8;13)(p12;q12). Br J Haematol. 2003;121:312–4.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Demiroglu A, Steer EJ, Heath C, Taylor K, Bentley M, Allen SL, et al. The t(8;22) in chronic myeloid leukemia fuses BCR to FGFR1: transforming activity and specific inhibition of FGFR1 fusion proteins. Blood. 2001;98:3778–83.
Montenegro-Garreaud X, Miranda RN, Reynolds A, Tang G, Wang SA, Yabe M, et al. Myeloproliferative neoplasms with t(8;22)(p11.2;q11.2)/BCR-FGFR1: a meta-analysis of 20 cases shows cytogenetic progression with B-lymphoid blast phase. Hum Pathol. 2017;65:147–56.
Gupta V, Kennedy JA, Capo-Chichi JM, Kim S, Hu ZH, Alyea EP, et al. Genetic factors rather than blast reduction determine outcomes of allogeneic HCT in BCR-ABL-negative MPN in blast phase. Blood Adv. 2020;4:5562–73.
Acknowledgements
JCHB, AP, LG, TC, DM and IYA were involved in study design, analysis and writing of the paper. NZ was the data manager. All other co-authors contributed data to the study, critically revised the paper and approved the submitted and final version.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
All authors declare no competing financial interests. This study was not economically funded. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. This retrospective study was approved by the Chronic Malignancies Working Party (CMWP) of EBMT. Informed consent for inclusion in the EBMT registry was obtained in all patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Two members from that institution collaborated in our study but we didn’t choose the preferred one for the final version of the manuscript. The suggested coauthor is Dr Emmanouil Nikolousis (manos.nikolousis@nhs.net) in place of Shankara Paneesha (shankara.paneesha@nhs.net). Both of them have agreed on this change.
Supplementary information
Rights and permissions
About this article
Cite this article
Hernández-Boluda, JC., Pereira, A., Zinger, N. et al. Allogeneic hematopoietic cell transplantation in patients with myeloid/lymphoid neoplasm with FGFR1-rearrangement: a study of the Chronic Malignancies Working Party of EBMT. Bone Marrow Transplant 57, 416–422 (2022). https://doi.org/10.1038/s41409-021-01553-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01553-x
This article is cited by
-
Myeloid/lymphoid neoplasm with eosinophilia and BCR/FGFR1 rearrangement with transformation to cortical T-lymphoblastic lymphoma and erythroid precursors: a case report
Journal of Medical Case Reports (2023)
-
Myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusions: reevaluation of the defining characteristics in a registry-based cohort
Leukemia (2023)
-
Updates on eosinophilic disorders
Virchows Archiv (2023)
-
Antineoplastics
Reactions Weekly (2022)