Abstract
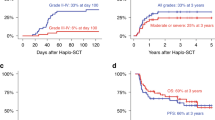
Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) is an aggressive hematological malignancy; however, some patients achieve durable remission with allogeneic hematopoietic cell transplantation (allo-HCT). We report on all 17 patients with BPDCN who underwent allo-HCT at our center between 2000 and 2020. The median age was 39 (18–67) years. All (n = 16, 94%), except one patient, had systemic disease involving bone marrow and/or other organs. Ten patients (59%) were in first complete remission (CR1) at allo-HCT. The donor source was matched related or unrelated in ten (59%) and alternate donor in seven (41%) patients. Five (31%) patients developed acute graft-versus-host disease (GVHD), all grade I-II. The cumulative incidence (CI) of chronic GVHD at five-year was 34%. The CI of non-relapse mortality at one-year was 29%. Progression-free survival (PFS) rates at two-year and five-year were 49% (95% CI = 22–71%) and 39% (95% CI = 14–64%), respectively. The two-year and five-year overall survival (OS) rates were 65% (95% CI = 38–82%) and 40% (95% CI = 12–68%), respectively. The five-year rate for both PFS and OS was 80% in CR1 patients versus 0% in patients not in CR1. In conclusion, allo-HCT provides long-lasting remissions in BPDCN patients, particularly when performed in CR1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Guru Murthy GS, Pemmaraju N, Atallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21–3.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405.
Deconinck E, Petrella T, Garnache Ottou F. Blastic Plasmacytoid Dendritic Cell Neoplasm: Clinical Presentation and Diagnosis. Hematol Oncol Clin North Am. 2020;34:491–500.
Togami K, Chung SS, Madan V, Kenyon CM, Cabal-Hierro L, Taylor J, et al. Sex-biased <em>ZRSR2</em> mutations in myeloid malignancies impair plasmacytoid dendritic cell activation and apoptosis. bioRxiv. 2020:2020.10.29.360503.
Sapienza MR, Fuligni F, Agostinelli C, Tripodo C, Righi S, Laginestra MA, et al. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 2014;28:1606–16.
Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356.
Beird HC, Khan M, Wang F, Alfayez M, Cai T, Zhao L, et al. Features of non-activation dendritic state and immune deficiency in blastic plasmacytoid dendritic cell neoplasm (BPDCN). Blood Cancer J 2019;9:99.
Alayed K, Patel KP, Konoplev S, Singh RR, Routbort MJ, Reddy N, et al. TET2 mutations, myelodysplastic features, and a distinct immunoprofile characterize blastic plasmacytoid dendritic cell neoplasm in the bone marrow. Am J Hematol. 2013;88:1055–61.
Pemmaraju N, Konopleva M. Approval of tagraxofusp-erzs for blastic plasmacytoid dendritic cell neoplasm. Blood Adv. 2020;4:4020–7.
Wang W, Khoury JD, Miranda RN, Jorgensen JL, Xu J, Loghavi S, et al. Immunophenotypic characterization of reactive and neoplastic plasmacytoid dendritic cells permits establishment of a 10-color flow cytometric panel for initial workup and residual disease evaluation of blastic plasmacytoid dendritic cell neoplasm. Haematologica. 2020.
Yun S, Chan O, Kerr D, Vincelette ND, Idrees A, Mo Q, et al. Survival outcomes in blastic plasmacytoid dendritic cell neoplasm by first-line treatment and stem cell transplant. Blood Adv. 2020;4:3435–42.
Sukswai N, Aung PP, Yin CC, Li S, Wang W, Wang SA, et al. Dual Expression of TCF4 and CD123 Is Highly Sensitive and Specific For Blastic Plasmacytoid Dendritic Cell Neoplasm. Am J Surg Pathol. 2019;43:1429–37.
Pemmaraju N, Konopleva M, Lane AA. More on Blastic Plasmacytoid Dendritic-Cell Neoplasms. N. Engl J Med. 2019;380:695–6.
Pagano L, Valentini CG, Pulsoni A, Fisogni S, Carluccio P, Mannelli F, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica 2013;98:239–46.
Taylor J, Haddadin M, Upadhyay VA, Grussie E, Mehta-Shah N, Brunner AM, et al. Multicenter analysis of outcomes in blastic plasmacytoid dendritic cell neoplasm offers a pretargeted therapy benchmark. Blood 2019;134:678–87.
Martin-Martin L, Lopez A, Vidriales B, Caballero MD, Rodrigues AS, Ferreira SI, et al. Classification and clinical behavior of blastic plasmacytoid dendritic cell neoplasms according to their maturation-associated immunophenotypic profile. Oncotarget 2015;6:19204–16.
Pemmaraju N, Lane AA, Sweet KL, Stein AS, Vasu S, Blum W, et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N. Engl J Med. 2019;380:1628–37.
Roos-Weil D, Dietrich S, Boumendil A, Polge E, Bron D, Carreras E, et al. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood 2013;121:440–6.
Aoki T, Suzuki R, Kuwatsuka Y, Kako S, Fujimoto K, Taguchi J, et al. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood 2015;125:3559–62.
Kharfan-Dabaja MA, Al Malki MM, Deotare U, Raj RV, El-Jurdi N, Majhail N, et al. Haematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: a North American multicentre collaborative study. Br J Haematol. 2017;179:781–9.
Leclerc M, Peffault de Latour R, Michallet M, Blaise D, Chevallier P, Rohrlich PS, et al. Can a reduced-intensity conditioning regimen cure blastic plasmacytoid dendritic cell neoplasm? Blood 2017;129:1227–30.
Kharfan-Dabaja MA, Reljic T, Murthy HS, Ayala E, Kumar A. Allogeneic Hematopoietic Cell Transplantation Is an Effective Treatment for Blastic Plasmacytoid Dendritic Cell Neoplasm in First Complete Remission: Systematic Review and Meta-analysis. Clin Lymphoma Myeloma Leuk. 2018;18:703–9 e1.
Reimer P, Rudiger T, Kraemer D, Kunzmann V, Weissinger F, Zettl A, et al. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transpl. 2003;32:637–46.
Laribi K, Baugier de Materre A, Sobh M, Cerroni L, Valentini CG, Aoki T, et al. Blastic plasmacytoid dendritic cell neoplasms: results of an international survey on 398 adult patients. Blood Adv. 2020;4:4838–48.
Author information
Authors and Affiliations
Contributions
QB, MHQ, and NP designed the study, interpreted the data, and wrote the manuscript; DRM analyzed the data and created figures; URP, PK, CH, IFK, KR, YN, BO, SS, NS, ALO, SA, GA, GR, MYK, REC, and EJS contributed to data collection, writing, and revision of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
QB has research funding from Acrotech and Stemline. QB served on advisory board of Purdue and Spectrum. MQ has research funding from Janssen, Bioline, Angiocrine, Amgen and Neximmune. SA has served on an advisory board and received research funding from Tessa Therapeutics and SeaGen. PK has research support from Amgen and Ziopharm; has served on advisory boards for Pfizer, Kite and Novartis; consulting fees from Jazz. MK has received grants and other from AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, and Forty-Seven. MK has received grants from Eli Lilly, Cellectis, Calithera, Ablynx, Agios, Ascentage, Astra Zeneca, Rafael Pharmaceutical and Sanofi. MK has received other from Amgen, Kisoji and Reata Pharmaceutical. MK holds US patent 7,795,305 B2, “CDDO-compounds and combination therapies thereof” with royalties paid to Reata Pharmaceutical, a patent Combination Therapy with a mutant IDH1 Inhibitor and a BCL-2 licensed to Eli Lilly, and patent 62/993,166 Combination of a MCL-1 Inhibitor And Midostaurin, Uses And Pharmaceutical Composition Thereof pending to Novartis. ES has received consultant fees and honoraria from Bayer HealthCare Pharmaceuticals, Novartis, Magenta and Adaptimmune. ES has received honoraria from Partner Therapeutics, Mesoblast and Axio. ES is the co-inventor on a provisional patent application owned by MD Anderson and licensed to Takeda. All other authors report no COI. This research was supported in part by the National Institutes of Health through the University of Texas MD Anderson Cancer Center’s Support Grant (CA016672).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bashir, Q., Milton, D.R., Popat, U.R. et al. Allogeneic hematopoietic cell transplantation for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN). Bone Marrow Transplant 57, 51–56 (2022). https://doi.org/10.1038/s41409-021-01478-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01478-5
This article is cited by
-
Diagnostic management of blastic plasmacytoid dendritic cell neoplasm (BPDCN) in close interaction with therapeutic considerations
Annals of Hematology (2024)
-
Abnormal karyotype is an independent predictor of inferior survival in Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN)
Blood Cancer Journal (2023)
-
Blastic plasmacytoid dendritic cell neoplasm: a comprehensive review in pediatrics, adolescents, and young adults (AYA) and an update of novel therapies
Leukemia (2023)