Abstract
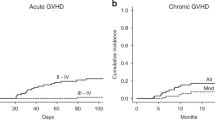
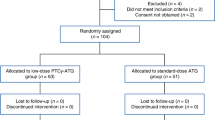
Antithymocyte globulin (ATG) has been shown to reduce chronic graft-versus-host disease (GVHD) particularly in allogeneic peripheral blood stem cell transplantation (PBSCT) from unrelated donors; however, anti-GVHD effects of lower doses of ATG remains to be elucidated. We conducted a nationwide retrospective study to compare the outcomes of unrelated PBSCT with or without rabbit ATG (thymoglobulin) in 287 patients. A median ATG dose was 2.0 mg/kg. The primary endpoint, the cumulative incidence of moderate–severe chronic GVHD at 2 years was 22.1% in the ATG group, which was significantly less than that in the non-ATG group (36.3%, P = 0.025). The ATG group had a higher incidence of immunosuppressant discontinuation, GVHD-free, relapse-free survival, and moderate–severe chronic GVHD-free, relapse-free survival at 2 years compared to the non-ATG group. The incidences of grade III–IV aGVHD and moderate–severe chronic GVHD were significantly higher in patients with high absolute lymphocyte count (ALC) before the administration of ATG, whereas relapse rate was significantly higher in patients with low ALC before ATG. In conclusion, low-dose ATG effectively suppresses chronic GVHD in unrelated PBSCT, and ALC before ATG may be a potential predictor for GVHD and relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY, et al. Long-term outcome and late effects in patients transplantedwith mobilised blood or bone marrow: a randomised trial. Lancet Oncol. 2010;11:331–8.
Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N. Engl J Med. 2012;367:1487–96.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Solh M, Zhang X, Connor K, Brown S, Solomon SR, Morris LE, et al. Factors predicting graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation: multivariable analysis from a single center. Biol Blood Marrow Transpl. 2016;22:1403–9.
Inamoto Y, Kimura F, Kanda J, Sugita J, Ikegame K, Nakasone H, et al. Comparison of graft-versus-host disease-free, relapse-free survival according to a variety of graft sources: antithymocyte globulin and single cord blood provide favorable outcomes in some subgroups. Haematologica. 2016;101:1592–602.
Zheng CC, Zhu XY, Tang BL, Zhang XH, Zhang L, Geng LQ, et al. Clinical separation of cGvHD and GvL and better GvHD-free/relapse-free survival (GRFS) after unrelated cord blood transplantation for AML. Bone Marrow Transpl. 2017;52:88–94.
Penack OL, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the european society for blood and marrow transplantation. Lancet Haematol. 2020;7:e157–67.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N. Engl J Med. 2016;374:43–53.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73.
Finke J, Schmoor C, Bethge WA, Ottinger H, Stelljes M, Volin L, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-t-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017;4:e293–301.
Bonifazi F, Solano C, Wolschke C, Sessa M, Patriarca F, Zallio F, et al. Acute GVHD prophylaxis plus ATLG after myeloablative allogeneic haemopoietic peripheral blood stem-cell transplantation from HLA-identical siblings in patients with acute myeloid leukaemia in remission: final results of quality of life and long-term outcome analysis of a phase 3 randomised study. Lancet Haematol. 2019;6:e89–99.
Baron F, Galimard JE, Labopin M, Yakoub-Agha I, Niittyvuopio R, Kröger N, et al. Allogeneic peripheral blood stem cell transplantation with anti-thymocyte globulin versus allogeneic bone marrow transplantation without anti-thymocyte globulin. Haematologica. 2020;105:1138–46.
Admiraal R, Nierkens S, de Witte MA, Petersen EJ, Fleurke GJ, Verrest L, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017;4:e183–91.
Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase III clinical trial of Anti-T-Lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35:4003–11.
Kennedy VE, Chen H, Savani BN, Greer J, Kassim AA, Engelhardt BG, et al. Optimizing antithymocyte globulin dosing for unrelated donor allogeneic hematopoietic cell transplantation based on recipient absolute lymphocyte count. Biol Blood Marrow Transpl. 2018;24:150–5.
Woo GU, Hong J, Kim H, Byun JM, Koh Y, Shin DY, et al. Preconditioning absolute lymphocyte count and transplantation outcomes in matched related donor allogeneic hematopoietic stem cell transplantation recipients with reduced-intensity conditioning and antithymocyte globulin treatment. Biol Blood Marrow Transpl. 2020;26:1855–60.
Modi D, Kim S, Surapaneni M, Ayash L, Ratanatharathorn V, Uberti JP, et al. Absolute lymphocyte count on the first day of thymoglobulin predicts relapse-free survival in matched unrelated peripheral blood stem cell transplantation. Leuk Lymphoma 2020;61:3137–45.
Heelan F, Mallick R, Bryant A, Radhwi O, Atkins H, Huebsch L, et al. Does lymphocyte count impact dosing of anti-thymocyte globulin in unrelated donor stem cell transplantation? Biol Blood Marrow Transpl. 2020;26:1298–302.
Jullien M, Guillaume T, Peterlin P, Garnier A, Le Bourgeois A, Debord C, et al. Antithymocyte globulin administration in patients with profound lymphopenia receiving a PBSC purine analog/busulfan-based conditioning regimen allograft. Sci Rep. 2020;10:15399.
Atsuta Y. Introduction of transplant registry unified management program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103:3–10.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–56.
Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110:4606–13.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Sheth V, Kennedy V, de Lavallade H, Mclornan D, Potter V, Engelhardt BG, et al. Differential interaction of peripheral blood lymphocyte counts (ALC) with different in vivo depletion strategies in predicting outcomes of allogeneic transplant: an international 2 center experience. Front Oncol. 2019;9:623.
Shichijo T, Fuji S, Nagler A, Bazarbachi A, Mohty M, Savani BN. Personalizing rabbit anti-thymocyte globulin therapy for prevention of graft-versus-host disease after allogeneic hematopoietic cell transplantation: is there an optimal dose? Bone Marrow Transpl. 2020;55:505–22.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98:2942–7.
Basara N, Baurmann H, Kolbe K, Yaman A, Labopin M, Burchardt A, et al. Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transpl. 2005;35:1011–8.
Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transpl. 2006;12:560–5.
Deeg HJ, Storer BE, Boeckh M, Martin PJ, McCune JS, Myerson D, et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transpl. 2006;12:573–84.
Bredeson CN, Zhang MJ, Agovi MA, Bacigalupo A, Bahlis NJ, Ballen K, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:993–1003.
Call SK, Kasow KA, Barfield R, Madden R, Leung W, Horwitz E, et al. Total and active rabbit antithymocyte globulin (rATG;Thymoglobulin) pharmacokinetics in pediatric patients undergoing unrelated donor bone marrow transplantation. Biol Blood Marrow Transpl. 2009;15:274–8.
Kim HJ, Min WS, Cho BS, Eom KS, Kim YJ, Min CK, et al. Successful prevention of acute graft-versus-host disease using low-dose antithymocyte globulin after mismatched, unrelated, hematopoietic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transpl. 2009;15:704–17.
Busca A, Locatelli F, Flonta SE, Ciccone G, Baldi I, D’Ardia S, et al. In vivo T-cell depletion with pretransplant low-dose antithymocyte globulin is associated with reduced transplant-related mortality and improved clinical outcome in patients receiving allogeneic hematopoietic stem cell transplantation from unrelated and partially matched related donors. Am J Hematol. 2011;86:214–7.
Bashir Q, Munsell MF, Giralt S, de Padua Silva L, Sharma M, Couriel D, et al. Randomized phase II trial comparing two dose levels of thymoglobulin in patients undergoing unrelated donor hematopoietic cell transplant. Leuk Lymphoma. 2012;53:915–9.
Al-Kadhimi Z, Gul Z, Rodriguez R, Chen W, Smith D, Mitchell A, et al. Anti-thymocyte globulin (thymoglobulin), tacrolimus, and sirolimus as acute graft-versus-host disease prophylaxis for unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2012;18:1734–44.
Kuriyama K, Fuji S, Inamoto Y, Tajima K, Tanaka T, Inoue Y, et al. Impact of low-dose rabbit anti-thymocyte globulin in unrelated hematopoietic stem cell transplantation. Int J Hematol. 2016;103:453–60.
Rubio MT, D’Aveni-Piney M, Labopin M, Hamladji RM, Sanz MA, Blaise D, et al. Impact of in vivo T cell depletion in HLA-identical allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission conditioned with a fludarabine iv-busulfan myeloablative regimen: a report from the EBMT Acute Leukemia Working Party. J Hematol Oncol. 2017;10:31.
Imataki O, Matsumoto K, Uemura M. Low-dose anti-thymocyte globulin reduce severe acute and chronic graft-versus-host disease after allogeneic stem cell transplantation. J Cancer Res Clin Oncol. 2017;143:709–15.
Bryant A, Mallick R, Huebsch L, Allan D, Atkins H, Anstee G, et al. Low-dose antithymocyte globulin for graft-versus-host-disease prophylaxis in matched unrelated allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2017;23:2096–101.
Shichijo T, Fuji S, Tajima K, Kubo H, Nozaki K, Honda T, et al. Beneficial impact of low-dose rabbit anti-thymocyte globulin in unrelated hematopoietic stem cell transplantation: focusing on difference between stem cell sources. Bone Marrow Transpl. 2018;53:634–9.
Tandra A, Covut F, Cooper B, Creger R, Brister L, McQuigg B, et al. Low dose anti-thymocyte globulin reduces chronic graft-versus-host disease incidence rates after matched unrelated donor transplantation. Leuk Lymphoma. 2018;59:1644–51.
Mountjoy L, Jain T, Kunze KL, Khera N, Sproat LZ, Jennifer W, et al. Clinical outcomes with low dose anti-thymocyte globulin in patients undergoing matched unrelated donor allogeneic hematopoietic cell transplantation. Leuk Lymphoma. 2020;61:1996–2002.
Chang YJ, Wu DP, Lai YR, Liu QF, Liu QF, Sun YQ, et al. Antithymocyte globulin for matched sibling donor transplantation in patients with hematologic malignancies: a multicenter, open-label, randomized controlled study. J Clin Oncol. 2020;38:3367–76.
Shiratori S, Kosugi-Kanaya M, Hayase E, Okada K, Goto H, Sugita J, et al. T-cell depletion effects of low-dose antithymocyte globulin for GVHD prophylaxis in HLA-matched allogeneic peripheral blood stem cell transplantation. Transpl Immunol. 2018;46:21–2.
Shiratori S, Sugita J, Ota S, Kasahara S, Ishikawa J, Tachibana T, et al. Low-dose anti-thymocyte globulin for GVHD prophylaxis in HLA-matched allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2021;56:129–36.
Shiratori S, Ohigashi H, Ara T, Yasumoto A, Goto H, Nakagawa M, et al. High lymphocyte counts before antithymocyte globulin administration predict acute graft-versus-host disease. Ann Hematol. 2021;100:1321–8.
Acknowledgements
We thank all the physicians and data managers who contributed valuable data to Transplant Registry Unified Management Program, especially those who participated in the additional survey. They also thank the staff of the Japanese Data Center for Hematopoietic Cell Transplantation for their assistance. This study was collaborated by HLA-WG and GVHD-WG in JSHCT. This study was supported by the Japan Agency for Medical Research and Development (AMED, 20ek0510025h0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SF reports grants and personal fees from CSL Behring, outside the submitted work. MS reports personal fees from Chugai, Pfizer, Astellas, Nippon-Shinyaku, Ono, MSD, Bristol-Myers Squibb, Kyowa-Hakko Kirin, Asahi-Kasei, Novartis, Eisai, Otsuka, Sumitomo Dainippon, Sanofi, Takeda, Celgene, Mochida, Shire, Mundipharma, outside the submitted work. KM reports personal fees from Kyowa Kirin Co. Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co. Ltd., Novartis Pharma Inc., Bristol-Myers Squibb, Ono Pharmaceutical Co. Ltd., MSD K.K., JIMRO Co. Ltd., outside the submitted work. KI reports personal fees from Chugai Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Novartis Pharma K.K., Celgene Co. Ltd., Bristol-Myers Squibb K.K., Takeda Pharmaceutical Co. Ltd., Nippon Shinyaku Co. Ltd., Otuka Pharmaceutical Co. Ltd., Astellas Pharma Inc., outside the submitted work. TI reports other from Astellas Pharma, Chugai Pharmaceutical Co., CSL Behring, Eisai Co., FUJIFILM Wako Chemicals., Kyowa Kirin Co., Ono Pharmaceutical Co., Pfizer, Nippon Shinyaku Co., MSD, Otsuka Pharmaceutical Co., Repertoire Genesis Inc., Sumitomo Dainippon Pharma Co., Taiho Pharmaceutical Co., other from Takara Bio Inc., Takeda Pharmaceutical Co., Zenyaku Kogyo Co., personal fees from Bristol-Myers Squibb, Celgene, personal fees from Janssen Pharmaceutical K.K., Kyowa Kirin Co., outside the submitted work. ST reports personal fees from Chugai Pharmaceutical Co. Ltd., Yakult Honsha Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Sumitomo Dainippon Pharma, Astellas Pharma Inc., Novartis, Amgen Astellas BioPharma K. K., outside the submitted work. YA reports grants from AMED, during the conduct of the study; other from Astellas Pharma Inc., Mochida Pharmaceutical Co. Ltd., Meiji Seika Pharma Co. Ltd., Chugai Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd, outside the submitted work. TT reports personal fees from Merck Sharp & Dohme, grants and personal fees from Kyowa Kirin, personal fees from Takeda, grants, personal fees, and non-financial support from Novartis, personal fees from Pfizer, Bristol-Myers Squibb, grants from Chugai, Sanofi, Astellas, Teijin Pharma, Fuji Pharma, Nippon Shinyaku, non-financial support from Janssen, grants from Japan Society for the Promotion of Science KAKENHI (17H04206), The Center of Innovation Program from Japan Science and Technology Agency, during the conduct of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shiratori, S., Sugita, J., Fuji, S. et al. Low-dose antithymocyte globulin inhibits chronic graft-versus-host disease in peripheral blood stem cell transplantation from unrelated donors. Bone Marrow Transplant 56, 2231–2240 (2021). https://doi.org/10.1038/s41409-021-01314-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01314-w
This article is cited by
-
Different effects of thymoglobulin on acute leukemia with pre-transplant residual blasts in HLA mismatch transplantation
International Journal of Hematology (2023)