Abstract
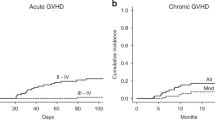
Antithymocyte globulin (ATG) and anti-T lymphocyte globulin (ATLG) have been widely used to prevent graft-versus-host disease (GvHD), each with distinct properties and noninterchangeable doses. However, the optimal dose of ATG in children undergoing allo-PBSCT for leukemia has not yet been established. Therefore, the impact of ATG dose on overall survival (OS), relapse, GvHD, and infectious complications was investigated. Patients administered high dose (unrelated: 7.5 mg/kg, haploidentical: 10.0 mg/kg) and low dose (unrelated: 3.75 mg/kg, haploidentical: 5.0 mg/kg) ATG during two consecutive time periods were compared. There were 78 (39.8%) patients in the low dose group and 118 (60.2%) in the high dose group. OS was superior in the low dose group compared to the high dose group (P = 0.017), and relapse incidence was significantly lower in the low dose group (P = 0.022). Cumulative incidences of acute and chronic GvHD were similar between the groups (P = 0.095 and P = 0.672, respectively). Cytomegalovirus reactivation (70.3% vs. 51.3%, P = 0.007), Epstein–Barr virus reactivation (81.4% vs. 39.7%, P < 0.001), and invasive bacterial infections (12.7% vs. 0%, P = 0.001) post transplant were more frequent in the high dose group compared to the low dose group. Therefore, low dose ATG is more optimal in pediatric allo-PBSCT providing better OS while lowering the risk of relapse and infectious complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bacigalupo A. Antilymphocyte/thymocyte globulin for graft versus host disease prophylaxis: efficacy and side effects. Bone Marrow Transplant. 2005;35:225–31.
Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–5.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–82.
Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–94.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98:2942–7.
Locatelli F, Bernardo ME, Bertaina A, Rognoni C, Comoli P, Rovelli A, et al. Efficacy of two different doses of rabbit anti-T-lymphocyte globulin to prevent graft-versus-host disease in children with haematological malignancies transplanted from an unrelated donor: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1126–36.
Oostenbrink LVE, Jol-van der Zijde CM, Kielsen K, Jansen-Hoogendijk AM, Ifversen M, Müller KG, et al. Differential elimination of anti-thymocyte globulin of fresenius and genzyme impacts T-cell reconstitution after hematopoietic stem. Cell Transplant. 2019;10:315.
Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TC, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015;2:e194–203.
Hashmi SK. Individualizing optimal dosing of antithymocyte globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24:2–3.
Bonifazi F, Rubio MT, Bacigalupo A, Boelens JJ, Finke J, Greinix H, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transplant. 2020;55:1093–102.
Lee JW, Kim SK, Jang PS, Chung NG, Jeong DC, Cho B, et al. Impact of CD34+ cell dose in children who receive unrelated PBSCT with in vivo T-cell depletion for hematologic malignancies. Bone Marrow Transplant. 2015;50:68–73.
Chung NG, Lee JW, Jang PS, Jeong DC, Cho B, Kim HK. Reduced dose cyclophosphamide, fludarabine and antithymocyte globulin for sibling and unrelated transplant of children with severe and very severe aplastic anemia. Pediatr Transpl. 2013;17:387–93.
Alexander S, Pole JD, Gibson P, Lee M, Hesser T, Chi SN, et al. Classification of treatment-related mortality in children with cancer: a systematic assessment. Lancet Oncol. 2015;16:e604–10.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transplant. 2016;22:4–10.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Baker M, Wang H, Rowley SD, Cai L, Pecora AL, Skarbnik A, et al. Comparative outcomes after haploidentical or unrelated donor bone marrow or blood stem cell transplantation in adult patients with hematological malignancies. Biol Blood Marrow Transplant. 2016;22:2047–55.
Choi SM, Lee DG, Lim J, Park SH, Choi JH, Yoo JH, et al. Comparison of quantitative cytomegalovirus real-time PCR in whole blood and pp65 antigenemia assay: clinical utility of CMV real-time PCR in hematopoietic stem cell transplant recipients. J Korean Med Sci. 2009;24:571–8.
Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2016;64:87–91.
Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, et al. Management of Epstein–Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803–11.
Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2019;71:1367–76.
Theurich S, Fischmann H, Shimabukuro-Vornhagen A, Chemnitz JM, Holtick U, Scheid C, et al. Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD009159.
Bryant A, Mallick R, Huebsch L, Allan D, Atkins H, Anstee G, et al. Low-dose antithymocyte globulin for graft-versus-host-disease prophylaxis in matched unrelated allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23:2096–101.
Baron F, Mohty M, Blaise D, Socié G, Labopin M, Esteve J, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:224–34.
Seidel MG, Fritsch G, Matthes-Martin S, Lawitschka A, Lion T, Potschger U, et al. Antithymocyte globulin pharmacokinetics in pediatric patients after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2005;27:532–6.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73.
Devillier R, Labopin M, Chevallier P, Ledoux MP, Socie G, Huynh A, et al. Impact of antithymocyte globulin doses in reduced intensity conditioning before allogeneic transplantation from matched sibling donor for patients with acute myeloid leukemia: a report from the acute leukemia working party of European group of Bone Marrow Transplantation. Bone Marrow Transplant. 2018;53:431–7.
Dabas R, Lee R, Servito MT, Dharmani-Khan P, Modi M, van Slyke T, et al. Antithymocyte globulin at clinically relevant concentrations kills leukemic blasts. Biol Blood Marrow Transplant. 2016;22:815–24.
Dabas R, Khan PD, Modi M, Khan FM, Storek J. More acute lymphoid leukemia than acute myeloid leukemia blasts are killed by rabbit antithymocyte globulin. Cytotherapy. 2019;21:1161–5.
Noviello M, Manfredi F, Ruggiero E, Perini T, Oliveira G, Cortesi F, et al. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat Commun. 2019;10:1065.
Chang YJ, Wang Y, Mo XD, Zhang XH, Xu LP, Yan CH, et al. Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: long-term outcomes of a prospective randomized trial. Cancer. 2017;123:2881–92.
Bosch M, Dhadda M, Hoegh-Petersen M, Liu Y, Hagel LM, Podgorny P, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. 2012;14:1258–75.
Freund-Brown J, Chirino L, Kambayashi T. Strategies to enhance NK cell function for the treatment of tumors and infections. Crit Rev Immunol. 2018;38:105–30.
Locatelli F, Pende D, Falco M, Della Chiesa M, Moretta A, Moretta L. NK cells mediate a crucial graft-versus-leukemia effect in haploidentical-HSCT to cure high-risk acute leukemia. Trends Immunol. 2018;39:577–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kang, H.M., Kim, S.K., Lee, J.W. et al. Efficacy of low dose antithymocyte globulin on overall survival, relapse rate, and infectious complications following allogeneic peripheral blood stem cell transplantation for leukemia in children. Bone Marrow Transplant 56, 890–899 (2021). https://doi.org/10.1038/s41409-020-01121-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01121-9
This article is cited by
-
Individualized dose of anti-thymocyte globulin based on weight and pre-transplantation lymphocyte counts in pediatric patients: a single center experience
Bone Marrow Transplantation (2024)
-
Impact of anti-thymocyte globulin on survival outcomes in female-to-male allogeneic hematopoietic stem cell transplantation
Scientific Reports (2023)
-
Improving Outcomes with Haploidentical Stem Cell Transplantation [HaploSCT] in Children Using Post-transplant Cyclophosphamide: a Single Center Experience
Indian Journal of Hematology and Blood Transfusion (2023)
-
Clinical characteristics and viral load patterns in children with cytomegalovirus gastrointestinal disease after allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2021)