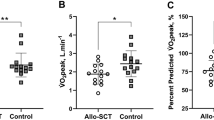
Abstract
Children with sickle cell disease (SCD) are at high-risk of progressive, chronic pulmonary and cardiac dysfunction. In this prospective multicenter Phase II trial of myeloimmunoablative conditioning followed by haploidentical stem cell transplantation in children with high-risk SCD, 19 patients, 2.0–21.0 years of age, were enrolled with one or more of the following: history of (1) overt stroke; (2) silent stroke; (3) elevated transcranial Doppler velocity; (4) multiple vaso-occlusive crises; and/or (5) two or more acute chest syndromes and received haploidentical transplants from 18 parental donors. Cardiac and pulmonary centralized cores were established. Pulmonary function results were expressed as percent of the median of healthy reference cohorts, matched for age, sex, height and race. At 2 years, pulmonary functions including forced expiratory volume (FEV), FEV1/ forced vital capacity (FVC), total lung capacity (TLC), diffusing capacity of lung for carbon monoxide (DLCO) were stable to improved compared to baseline values. Importantly, specific airway conductance was significantly improved at 2 years (p < 0.004). Left ventricular systolic function (fractional shortening) and tricuspid regurgitant velocity were stable at 2 years. These results demonstrate that haploidentical stem cell transplantation can stabilize or improve cardiopulmonary function in patients with SCD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Caughey MC, Poole C, Ataga KI, Hinderliter AL. Estimated pulmonary artery systolic pressure and sickle cell disease: a meta-analysis and systematic review. Br J Haematol. 2015;170:416–24.
Fitzhugh CD, Lauder N, Jonassaint JC, Telen MJ, Zhao X, Wright EC, et al. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85:36–40.
Gladwin MT. Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 2016;387:2565–74.
Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N. Engl J Med. 2004;350:886–95.
Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N. Engl J Med. 2008;359:2254–65.
Lanzkron S, Carroll CP, Haywood C Jr. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep. 2013;128:110–6.
MacLean JE, Atenafu E, Kirby-Allen M, MacLusky IB, Stephens D, Grasemann H, et al. Longitudinal decline in lung volume in a population of children with sickle cell disease. Am J Respir Crit Care Med. 2008;178:1055–9.
Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N. Engl J Med. 2017;376:1561–73.
Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl J Med. 1994;330:1639–44.
Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–9.
Steinberg MH. Management of sickle cell disease. N. Engl J Med. 1999;340:1021–30.
World Health Organization Regional Office for Africa. Sickle-cell disease: a strategy for the WHO African Region. Report of the Regional Director. Equatorial Guinea: WHO; 2010.
Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–51.
Piel FB, Tatem AJ, Huang Z, Gupta S, Williams TN, Weatherall DJ. Global migration and the changing distribution of sickle haemoglobin: a quantitative study of temporal trends between 1960 and 2000. Lancet Glob Health. 2014;2:e80–9.
Field JJ, Glassberg J, Gilmore A, Howard J, Patankar S, Yan Y, et al. Longitudinal analysis of pulmonary function in adults with sickle cell disease. Am J Hematol. 2008;83:574–6.
Klings ES, Wyszynski DF, Nolan VG, Steinberg MH. Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med. 2006;173:1264–9.
Koumbourlis AC. Lung function in sickle cell disease. Paediatr Respir Rev. 2014;15:33–7.
Field JJ, DeBaun MR, Yan Y, Strunk RC. Growth of lung function in children with sickle cell anemia. Pediatr Pulmonol. 2008;43:1061–6.
Koumbourlis AC, Zar HJ, Hurlet-Jensen A, Goldberg MR. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. J Pediatr. 2001;138:188–92.
Lunt A, McGhee E, Sylvester K, Rafferty G, Dick M, Rees D, et al. Longitudinal assessment of lung function in children with sickle cell disease. Pediatr Pulmonol. 2016;51:717–23.
Lunt A, Desai SR, Wells AU, Hansell DM, Mushemi S, Melikian N, et al. Pulmonary function, CT and echocardiographic abnormalities in sickle cell disease. Thorax. 2014;69:746–51.
Arteta M, Campbell A, Nouraie M, Rana S, Onyekwere OC, Ensing G, et al. Abnormal pulmonary function and associated risk factors in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol. 2014;36:185–9.
Lunt A, McGhee E, Robinson P, Rees D, Height S, Greenough A. Lung function, transfusion, pulmonary capillary blood volume and sickle cell disease. Respir Physiol Neurobiol. 2016;222:6–10.
Ruhl AP, Sadreameli SC, Allen JL, Bennett DP, Campbell AD, Coates TD, et al. Identifying clinical and research priorities in sickle cell lung disease. an official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2019;16:e17–e32.
Inamoto Y, Lee SJ. Late effects of blood and marrow transplantation. Haematologica. 2017;102:614–25.
Roca J, Granena A, Rodriguez-Roisin R, Alvarez P, Agusti-Vidal A, Rozman C. Fatal airway disease in an adult with chronic graft-versus-host disease. Thorax. 1982;37:77–8.
Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2011;17:1072–8.
Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2010;16:S106–14.
Cheng GS, Storer B, Chien JW, Jagasia M, Hubbard JJ, Burns L, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc. 2016;13:1932–9.
Ambrusko SJ, Gunawardena S, Sakara A, Windsor B, Lanford L, Michelson P, et al. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr Blood Cancer. 2006;47:907–13.
Genberg M, Oberg A, Andren B, Hedenstrom H, Frisk P, Flachskampf FA. Cardiac function after hematopoietic cell transplantation: an echocardiographic cross-sectional study in young adults treated in childhood. Pediatr Blood Cancer. 2015;62:143–7.
Rotz SJ, Dandoy CE, Taylor MD, Jodele S, Jefferies JL, Lane A, et al. Long-term systolic function in children and young adults after hematopoietic stem cell transplant. Bone Marrow Transpl. 2017;52:1443–7.
Rotz SJ, Ryan TD, Hayek SS. Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation. J Thromb Thrombolysis. 2020. https://doi.org/10.1007/s11239-020-02344-9. Online ahead of print.
Talano JA, Cairo MS. Smoothing the crescent curve: sickle cell disease. Hematol Am Soc Hematol Educ Program. 2014;2014:468–74.
Talano JA, Cairo MS. Hematopoietic stem cell transplantation for sickle cell disease: state of the science. Eur J Haematol. 2015;94:391–9.
Bernaudin F, Dalle JH, Bories D, de Latour RP, Robin M, Bertrand Y, et al. Long-term event-free survival, chimerism and fertility outcomes in 234 patients with sickle-cell anemia younger than 30 years after myeloablative conditioning and matched-sibling transplantation in France. Haematologica. 2020;105:91–101.
Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–56.
Bhatia M, Jin Z, Baker C, Geyer MB, Radhakrishnan K, Morris E, et al. Reduced toxicity, myeloablative conditioning with BU, fludarabine, alemtuzumab and SCT from sibling donors in children with sickle cell disease. Bone Marrow Transpl. 2014;49:913–20.
Gluckman E, Cappelli B, Bernaudin F, Labopin M, Volt F, Carreras J, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129:1548–56.
Guilcher GMT, Monagel DA, Nettel-Aguirre A, Truong TH, Desai SJ, Bruce A, et al. Nonmyeloablative matched sibling donor hematopoietic cell transplantation in children and adolescents with sickle cell disease. Biol Blood Marrow Transpl. 2019;25:1179–86.
Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–17.
King AA, Kamani N, Bunin N, Sahdev I, Brochstein J, Hayashi RJ, et al. Successful matched sibling donor marrow transplantation following reduced intensity conditioning in children with hemoglobinopathies. Am J Hematol. 2015;90:1093–8.
Locatelli F, Kabbara N, Ruggeri A, Ghavamzadeh A, Roberts I, Li CK, et al. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013;122:1072–8.
Walters MC, Patience M, Leisenring W, Eckman JR, Scott JP, Mentzer WC, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–76.
Mentzer WC, Heller S, Pearle PR, Hackney E, Vichinsky E. Availability of related donors for bone marrow transplantation in sickle cell anemia. Am J Pediatr Hematol Oncol. 1994;16:27–9.
Stenger EO, Shenoy S, Krishnamurti L. How I treat sickle cell disease with hematopoietic cell transplantation. Blood. 2019;134:2249–60.
Abraham A, Cluster A, Jacobsohn D, Delgado D, Hulbert ML, Kukadiya D, et al. Unrelated umbilical cord blood transplantation for sickle cell disease following reduced-intensity conditioning: results of a phase I trial. Biol Blood Marrow Transpl. 2017;23:1587–92.
Kamani NR, Walters MC, Carter S, Aquino V, Brochstein JA, Chaudhury S, et al. Unrelated donor cord blood transplantation for children with severe sickle cell disease: results of one cohort from the phase II study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN). Biol Blood Marrow Transpl. 2012;18:1265–72.
Ngwube A, Shah N, Godder K, Jacobsohn D, Hulbert ML, Shenoy S. Abatacept is effective as GVHD prophylaxis in unrelated donor stem cell transplantation for children with severe sickle cell disease. Blood Adv. 2020;4:3894–9.
Radhakrishnan K, Bhatia M, Geyer MB, Del Toro G, Jin Z, Baker C, et al. Busulfan, fludarabine, and alemtuzumab conditioning and unrelated cord blood transplantation in children with sickle cell disease. Biol Blood Marrow Transpl. 2013;19:676–7.
Shenoy S, Eapen M, Panepinto JA, Logan BR, Wu J, Abraham A, et al. A trial of unrelated donor marrow transplantation for children with severe sickle cell disease. Blood. 2016;128:2561–7.
Geyer MB, Ricci AM, Jacobson JS, Majzner R, Duffy D, Van de Ven C, et al. T cell depletion utilizing CD34(+) stem cell selection and CD3(+) addback from unrelated adult donors in paediatric allogeneic stem cell transplantation recipients. Br J Haematol. 2012;157:205–19.
Cairo MS, Talano JA, Moore TB, Shi Q, Weinberg RS, Grossman B, et al. Familial haploidentical stem cell transplant in children and adolescents with high-risk sickle cell disease: a phase 2 clinical trial. JAMA Pediatr. 2020;174:195–7.
Long BG, Flower A, Fabricatore S, Morris E, Mahanti H, Shi Q, et al. Assessment of safety and efficacy of PBSC mobilization with G-CSF and CD34+ enrichment and pbmnc (CD3+) addback in familial haploidentical (FHI) adult donors with sickle cell disease trait (SCDT) prior to allogeneic HSCT of high-risk SCD patients. Biol Blood Marrow Transplant. 2019;25:S310.
Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, et al. ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;2017:49.
Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200:e70–e88.
Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22.
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43.
Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35.
Mondal P, Yirinec A, Midya V, Sankoorikal BJ, Smink G, Khokhar A, et al. Diagnostic value of spirometry vs impulse oscillometry: a comparative study in children with sickle cell disease. Pediatr Pulmonol. 2019;54:1422–30.
Nyilas S, Baumeler L, Tamm M, Halter JP, Savic S, Korten I, et al. Inert gas washout in bronchiolitis obliterans following hematopoietic cell transplantation. Chest. 2018;154:157–68.
Verbanck S, Thompson BR, Schuermans D, Kalsi H, Biddiscombe M, Stuart-Andrews C, et al. Ventilation heterogeneity in the acinar and conductive zones of the normal ageing lung. Thorax. 2012;67:789–95.
Bussamra MH, Cukier A, Stelmach R, Rodrigues JC. Evaluation of the magnitude of the bronchodilator response in children and adolescents with asthma. Chest. 2005;127:530–5.
Tavares e Castro A, Matos P, Tavares B, Matos MJ, Segorbe-Luis A. Alternative functional criteria to assess airflow-limitation reversibility in asthma. Rev Port Pneumol. 2015;21:69–75.
Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics. 2008;121:777–82.
Morice AH, Waterhouse JC, Peers EM, Parry-Billings M. Use of whole-body plethysmography to compare bronchodilator inhaler efficacy. Respiration. 1998;65:120–4.
Skinner C, Palmer KN. Changes in specific airways conductance and forced expiratory volume in one second after a bronchodilator in normal subjects and patients with airways obstruction. Thorax. 1974;29:574–7.
Aurora P, Kozlowska W, Stocks J. Gas mixing efficiency from birth to adulthood measured by multiple-breath washout. Respir Physiol Neurobiol. 2005;148:125–39.
Usemann J, Yammine S, Singer F, Latzin P. Inert gas washout: background and application in various lung diseases. Swiss Med Wkly. 2017;147:w14483.
Gordeuk VR, Castro OL, Machado RF. Pathophysiology and treatment of pulmonary hypertension in sickle cell disease. Blood. 2016;127:820–8.
Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127:750–60.
Harrington JK, Krishnan U, Jin Z, Mardy C, Kobsa S, Lee MT. Longitudinal analysis of echocardiographic abnormalities in children with sickle cell disease. J Pediatr Hematol Oncol. 2017;39:500–5.
Badawy SM, Liem RI, Rigsby CK, Labotka RJ, DeFreitas RA, Thompson AA. Assessing cardiac and liver iron overload in chronically transfused patients with sickle cell disease. Br J Haematol. 2016;175:705–13.
Armenian SH, Ryan TD, Khouri MG. Cardiac dysfunction and heart failure in hematopoietic cell transplantation survivors: emerging paradigms in pathophysiology, screening, and prevention. Heart Fail Clin. 2017;13:337–45.
Covi S, Ravindranath Y, Farooqi A, Savasan S, Chu R, Aggarwal S. Changes in bi-ventricular function after hematopoietic stem cell transplant as assessed by speckle tracking echocardiography. Pediatr Cardiol. 2018;39:365–74.
Acknowledgements
This study was supported in large part by FDA R01FD004090 held by Dr. Cairo and in small part by the Pediatric Cancer Research Foundation, held by Dr. Cairo. We would like to thank Virginia Davenport, RN for her superb editorial assistance. We would also like to thank the patients and families who participated in this clinical trial and all the members of the external data safety monitoring committee and external advisory committee (Supplementary Fig. 3).
Funding
This study was supported in large part by FDA R01FD004090 held by MSC and in small part by the Pediatric Cancer Research Foundation, held by MSC.
Author information
Authors and Affiliations
Contributions
MSC, AJD, DF, MD, JM, QS and CV had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. MSC, JT, TBM, MCW and SS contributed to the concept and design of study. MSC, DF, AJD, MD, QS, CVV and JM collected, analyzed and interpreted the data. MSC, AJD, DF, MD, JM, CVV, and EM drafted the manuscript. MSC, AJD, DF, MD, JM, CVV and EM drafted the manuscript. MSC, AJD, DF, MD, JM, JT, TBM, SS, QS, MCW, EV, SP, SB, CM, JA, AF, EM, HM, SB, LK, CVV and LBL critically revised the manuscript for important intellectual content. QS and CVV provided the statistical analysis. MSC obtained the funding and Miltenyi generously provided the CD34 enriched reagents for this study. All authors contributed to the writing of this manuscript and have approved this submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MSC has received funding from the FDA (R01FD004090), the Pediatric Cancer Research Foundation (PCRF), Otsuka and Miltenyi. Although not related to this study, MCW is a consultant for Bluebird Bio, Inc., Bioverativ, Sangamo, Veevo, Editas Medicine and is medical director for AllCells, Inc. Biosciences. SKP is a consultant to Seattle Genetics, also unrelated to this study. EV is a consultant for ApoPharma, on the advisory committee for Global Blood Therapeutics and receives royalties from UptoDate. All other authors declare no conflicts of interest.
Ethics
The protocol was approved at each institutional review board, informed consent was obtained and assent was obtained if clinically applicable and the study was registered at ClinicalTrials.gov NCT01461837. This research has been presented in part at the American Society of Hematology (ASH), December 2017, San Diego, CA.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Friedman, D., Dozor, A.J., Milner, J. et al. Stable to improved cardiac and pulmonary function in children with high-risk sickle cell disease following haploidentical stem cell transplantation. Bone Marrow Transplant 56, 2221–2230 (2021). https://doi.org/10.1038/s41409-021-01298-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01298-7