Abstract
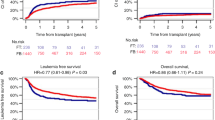
The impact of conditioning regimen prior to hematopoietic cell transplant (HCT) in pediatric AML-patients is not well studied. We retrospectively analyzed the impact of Busulfan–Cyclophosphamide (BuCy), Busulfan–Cyclophosphamide–Melphalan (BuCyMel) and Clofarabine–Fludarabine–Busulfan (CloFluBu) in pediatric AML-patients, with similar upfront leukemia treatment (NOPHO-DBHconsortium), receiving an HCT between 2010 and 2015. Outcomes of interest were LFS, relapse, TRM and GvHD. 103 patients were included; 30 received BuCy, 37 BuCyMel, and 36 CloFluBu. The 5-years LFS was 43.3% (SE ± 9.0) in the BuCy group, 59.2 % (SE ± 8.1) after BuCyMel, and 66.7 % (SE ± 7.9) after CloFluBu. Multivariable Cox regression analysis showed a trend to lower LFS after BuCy compared to CloFluBu (p = 0.07). BuCy was associated with a higher relapse incidence compared to the other regimens (p = 0.06). Younger age was a predictor for relapse (p = 0.02). A strong correlation between Busulfan Therapeutic Drug Monitoring (TDM) and lower incidence of aGvHD (p < 0.001) was found. In conclusion, LFS after BuCyMel and CloFluBu was comparable, lower LFS was found after BuCy, due to higher relapse incidence. CloFluBu was associated with lower incidence of aGvHD, suggesting lower toxicity with this type of conditioning. This finding is also explained by the impact of Busulfan monitoring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
15 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41409-021-01257-2
References
Andersson BS, Valdez BC, de Lima M, Wang X, Thall PF, Worth LL, et al. Clofarabine +/- fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant. 2011;17:893–900.
Alatrash G, Thall PF, Valdez BC, Fox PS, Ning J, Garber HR, et al. Long-term outcomes after treatment with clofarabine +/- fludarabine with once-daily intravenous busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2016;22:1792–800.
Fozza C. The role of Clofarabine in the treatment of adults with acute myeloid leukemia. Crit Rev Oncol Hematol. 2015;93:237–45.
Messinger Y, Boklan J, Goldberg J, DuBois SG, Oesterheld J, Abla O, et al. Combination of clofarabine, cyclophosphamide, and etoposide for relapsed or refractory childhood and adolescent acute myeloid leukemia. Pediatr Hematol Oncol. 2017;34:187–98.
van Eijkelenburg NKA, Rasche M, Ghazaly E, Dworzak MN, Klingebiel T, Rossig C, et al. Clofarabine, high-dose cytarabine and liposomal daunorubicin in pediatric relapsed/refractory acute myeloid leukemia: a phase IB study. Haematologica. 2018;103:1484–92.
Buttner B, Knoth H, Kramer M, Oertel R, Seeling A, Sockel K, et al. Impact of pharmacokinetics on the toxicity and efficacy of clofarabine in patients with relapsed or refractory acute myeloid leukemia. Leuk Lymphoma. 2017;58:2865–74.
Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–9.
Karlsson L, Forestier E, Hasle H, Jahnukainen K, Jonsson OG, Lausen B, et al. Outcome after intensive reinduction therapy and allogeneic stem cell transplant in paediatric relapsed acute myeloid leukaemia. Br J Haematol. 2017;178:592–602.
Hasle H. A critical review of which children with acute myeloid leukaemia need stem cell procedures. Br J Haematol. 2014;166:23–33.
Phillips GL, Shepherd JD, Barnett MJ, Lansdorp PM, Klingemann HG, Spinelli JJ, et al. Busulfan, cyclophosphamide, and melphalan conditioning for autologous bone marrow transplantation in hematologic malignancy. J Clin Oncol. 1991;9:1880–8.
Strahm B, Nollke P, Zecca M, Korthof ET, Bierings M, Furlan I, et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: results of the EWOG-MDS 98 study. Leukemia. 2011;25:455–62.
Sauer MG, Lang PJ, Albert MH, Bader P, Creutzig U, Eyrich M, et al. Hematopoietic stem cell transplantation for children with acute myeloid leukemia-results of the AML SCT-BFM 2007 trial. Leukemia. 2020;34:613–24.
Lucchini G, Labopin M, Beohou E, Dalissier A, Dalle JH, Cornish J, et al. Impact of conditioning regimen on outcomes for children with acute myeloid leukemia undergoing transplantation in first complete remission. An analysis on behalf of the Pediatric Disease Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23:467–74.
Samuels BL, Bitran JD. High-dose intravenous melphalan: a review. J Clin Oncol. 1995;13:1786–99.
Bayraktar UD, Bashir Q, Qazilbash M, Champlin RE, Ciurea SO. Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:344–56.
Hassan M, Oberg G, Ericson K, Ehrsson H, Eriksson L, Ingvar M, et al. In vivo distribution of [11C]-busulfan in cynomolgus monkey and in the brain of a human patient. Cancer Chemother Pharmacol. 1992;30:81–5.
Hassan M, Ehrsson H, Smedmyr B, Totterman T, Wallin I, Oberg G, et al. Cerebrospinal fluid and plasma concentrations of busulfan during high-dose therapy. Bone Marrow Transplant. 1989;4:113–4.
Yeager AM, Wagner JE Jr, Graham ML, Jones RJ, Santos GW, Grochow LB. Optimization of busulfan dosage in children undergoing bone marrow transplantation: a pharmacokinetic study of dose escalation. Blood. 1992;80:2425–8.
Philippe M, Neely M, Rushing T, Bertrand Y, Bleyzac N, Goutelle S. Maximal concentration of intravenous busulfan as a determinant of veno-occlusive disease: a pharmacokinetic-pharmacodynamic analysis in 293 hematopoietic stem cell transplanted children. Bone Marrow Transplant. 2019;54:448–57.
Bartelink IH, van Reij EM, Gerhardt CE, van Maarseveen EM, de Wildt A, Versluys B, et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014;20:345–53.
Zaucha-Prazmo A, Gozdzik J, Debski R, Drabko K, Sadurska E, Kowalczyk JR. Transplant-related mortality and survival in children with malignancies treated with allogeneic hematopoietic stem cell transplantation. A multicenter analysis. Pediatr Transplant. 2018;22:e13158.
Ishida Y, Honda M, Ozono S, Okamura J, Asami K, Maeda N, et al. Late effects and quality of life of childhood cancer survivors: part 1. Impact of stem cell transplantation. Int J Hematol. 2010;91:865–76.
Holmqvist AS, Chen Y, Wu J, Battles K, Bhatia R, Francisco L, et al. Assessment of late mortality risk after allogeneic blood or marrow transplantation performed in childhood. JAMA Oncol. 2018;4:e182453.
Acknowledgements
We would like to thank Statistikertjänst at Gothenburg University for excellent statistical analysis. We also want to thank doctors, nurses, and patients in the different centers for contributing to the study. The study was sponsored by Swedish Childhood Cancer Foundation and The Västra Götaland County Council (ALF project).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The spelling of J. Buechner’s and J. Abrahamsson’s names was incorrect.
Supplementary information
Rights and permissions
About this article
Cite this article
Versluys, A.B., Boelens, J.J., Pronk, C. et al. Hematopoietic cell transplant in pediatric acute myeloid leukemia after similar upfront therapy; a comparison of conditioning regimens. Bone Marrow Transplant 56, 1426–1432 (2021). https://doi.org/10.1038/s41409-020-01201-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01201-w