Abstract
The delayed recovery of adaptive immunity underlies transplant-related mortality (TRM) after αβ T cell-depleted hematopoietic stem cell transplantation (HSCT). We tested the use of low-dose memory donor lymphocyte infusions (mDLIs) after engraftment of αβ T cell-depleted grafts.
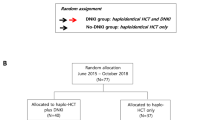
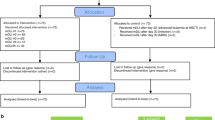
A cohort of 131 pediatric patients (median age 9 years) were grafted with αβ T cell-depleted products from either haplo (n = 79) or unrelated donors (n = 52). After engraftment, patients received mDLIs prepared by CD45RA depletion. Cell dose was escalated monthly from 25 × 103 to 100 × 103/kg (haplo) and from 100 × 103 to 300 × 103 /kg (MUD). In a subcohort of 16 patients, T-cell receptor (TCR) repertoire profiling with deep sequencing was used to track T-cell clones and to evaluate the contribution of mDLI to the immune repertoire.
In total, 343 mDLIs were administered. The cumulative incidence (CI) of grades II and III de novo acute graft-versus-host disease (aGVHD) was 5% and 2%, respectively, and the CI of chronic graft-versus-host disease was 7%. Half of the patients with undetectable CMV-specific T cells before mDLI recovered CMV-specific T cells. TCR repertoire profiling confirmed that mDLI-derived T cells significantly contribute to the TCR repertoire up to 1 year after HSCT and include persistent, CMV-specific T-cell clones.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chaleff S, Otto M, Barfield RC, Leimig T, Iyengar R, Martin J, et al. A large-scale method for the selective depletion of alphabeta T lymphocytes from PBSC for allogeneic transplantation. Cytotherapy. 2007;9:746–54. https://doi.org/10.1080/14653240701644000.
Handgretinger R. New approaches to graft engineering for haploidentical bone marrow transplantation. Semin Oncol. 2012;39:664–73. https://doi.org/10.1053/j.seminoncol.2012.09.007.
Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood. 2014;124:822–6. https://doi.org/10.1182/blood-2014-03-563817.
Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and B-cell depletion. Blood. 2017;130:677–85. https://doi.org/10.1182/blood-2017-04-779769.
Balashov D, Shcherbina A, Maschan M, Trakhtman P, Skvortsova Y, Shelikhova L, et al. Single-center experience of unrelated and haploidentical stem cell transplantation with TCRalphabeta and CD19 depletion in children with primary immunodeficiency syndromes. Biol Blood Marrow Transplant. 2015;21:1955–62. https://doi.org/10.1016/j.bbmt.2015.07.008.
Maschan M, Shelikhova L, Ilushina M, Kurnikova E, Boyakova E, Balashov D, et al. TCR-alpha/beta and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone Marrow Transplant. 2016;51:668–74. https://doi.org/10.1038/bmt.2015.343.
Laberko A, Sultanova E, Gutovskaya E, Shipitsina I, Shelikhova L, Kurnikova E, et al. Mismatched related vs. matched unrelated donors in TCRalphabeta/CD19-depleted HSCT for primary immunodeficiencies. Blood. 2019;134:1755–63. https://doi.org/10.1182/blood.2019001757.
Laberko A, Bogoyavlenskaya A, Shelikhova L, Shekhovtsova Z, Balashov D, Voronin K, et al. Risk factors for and the clinical impact of cytomegalovirus and Epstein–Barr virus infections in pediatric recipients of TCR-alpha/beta- and CD19-depleted grafts. Biol Blood Marrow Transplant. 2017;23:483–90. https://doi.org/10.1016/j.bbmt.2016.12.635.
Zvyagin IV, Mamedov IZ, Tatarinova OV, Komech EA, Kurnikova EE, Boyakova EV, et al. Tracking T-cell immune reconstitution after TCRalphabeta/CD19-depleted hematopoietic cells transplantation in children. Leukemia. 2017;31:1145–53. https://doi.org/10.1038/leu.2016.321.
Triplett BM, Shook DR, Eldridge P, Li Y, Kang G, Dallas M, et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplant. 2015;50:968–77.
Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Investig. 2015;125:2677–89. https://doi.org/10.1172/JCI81229.
Maschan M, Blagov S, Shelikhova L, Shekhovtsova Z, Balashov D, Starichkova J, et al. Low-dose donor memory T-cell infusion after TCR alpha/beta depleted unrelated and haploidentical transplantation: results of a pilot trial. Bone Marrow Transplant. 2018;53:264–73. https://doi.org/10.1038/s41409-017-0035-y.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56. https://doi.org/10.1016/j.bbmt.2005.09.004.
Kivioja T, Vaharautio A, Karlsson K, Bonke M, Enge M, Linnarsson S, et al. Counting absolute numbers of molecules using unique molecular identifiers. Nat Methods. 2011;9:72–4. https://doi.org/10.1038/nmeth.1778.
Egorov ES, Merzlyak EM, Shelenkov AA, Britanova OV, Sharonov GV, Staroverov DB, et al. Quantitative profiling of immune repertoires for minor lymphocyte counts using unique molecular identifiers. J Immunol. 2015;194:6155–63. https://doi.org/10.4049/jimmunol.1500215.
Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, et al. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci USA. 2014;111:13139–44. https://doi.org/10.1073/pnas.1409155111.
Bagaev DV, Vroomans RMA, Samir J, Stervbo U, Rius C, Dolton G, et al. VDJdb in 2019: database extension, new analysis infrastructure and a T-cell receptor motif compendium. Nucleic Acids Res. 2020;48:D1057–62. https://doi.org/10.1093/nar/gkz874.
Perruccio K, Sisinni L, Perez-Martinez A, Valentin J, Capolsini I, Massei MS, et al. High incidence of early human Herpesvirus-6 infection in children undergoing haploidentical manipulated stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2018;24:2549–57.
Sisinni L, Gasior M, de Paz R, Querol S, Bueno D, Fernandez L, et al. Unexpected high incidence of human herpesvirus-6 encephalitis after naive T cell-depleted graft of haploidentical stem cell transplantation in pediatric patients. Biol Blood Marrow Transplant. 2018;24:2316–23. https://doi.org/10.1016/j.bbmt.2018.07.016.
Keller MD, Darko S, Lang H, Ransier A, Lazarski CA, Wang Y, et al. T-cell receptor sequencing demonstrates persistence of virus-specific T cells after antiviral immunotherapy. Br J Haematol. 2019;187:206–18. https://doi.org/10.1111/bjh.16053.
Distler E, Bloetz A, Albrecht J, Asdufan S, Hohberger A, Frey M, et al. Alloreactive and leukemia-reactive T cells are preferentially derived from naive precursors in healthy donors: implications for immunotherapy with memory T cells. Haematologica. 2011;96:1024–32. https://doi.org/10.3324/haematol.2010.037481.
Chen BJ, Deoliveira D, Cui X, Le NT, Son J, Whitesides JF, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109:3115–23. https://doi.org/10.1182/blood-2006-04-016410.
Foster AE, Marangolo M, Sartor MM, Alexander SI, Hu M, Bradstock KF, et al. Human CD62L- memory T cells are less responsive to alloantigen stimulation than CD62L+ naive T cells: potential for adoptive immunotherapy and allodepletion. Blood. 2004;104:2403–9. https://doi.org/10.1182/blood-2003-12-4431.
Triplett BM, Muller B, Kang G, Li Y, Cross SJ, Moen J, et al. Selective T-cell depletion targeting CD45RA reduces viremia and enhances early T-cell recovery compared with CD3-targeted T-cell depletion. Transpl Infect Dis. 2018;20. https://doi.org/10.1111/tid.12823.
Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, et al. Age-Related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192:2689–98
Acknowledgements
The authors thank the physicians and nursing staff of the HSCT Units 1 and 2 for outstanding patient care, the staff of molecular biology, microbiology, and transplant biology laboratories for excellent service. We are grateful to the “Podari Zhizn” foundation and “Science for children” foundation for continued support of the care of the patients and research in the field of HSCT.
Funding
The study was supported by RFBR grant no. 18-34-20139 (IZ, EK, FV), MM, AM, EO, DC were partly supported by grant of the Ministry of Science and Higher Education of the Russian Federation no. 075-15-2020-807.
Author information
Authors and Affiliations
Contributions
MM, IZ, and SB contributed equally to this work. MM designed the study, analyzed data, and wrote the paper, IZ designed the sequencing part, collected and analyzed data, and wrote the paper, SB collected and analyzed data, wrote the paper, LS collected and analyzed data, reviewed the paper, RK, DB, JS, EK, DP, YM, AK, EO, MF, SG collected and analyzed data, EK collected samples, FV sample processing, data analysis, MS bioinformatics, data analysis, DC study design, data analysis, review, GN, AM—study design, paper review.
Corresponding author
Ethics declarations
Conflict of interest
MM received lecturer’s fee from Miltenyi Biotec, all other authors declare no conflict of interest in relation to the current paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Blagov, S., Zvyagin, I.V., Shelikhova, L. et al. T-cell tracking, safety, and effect of low-dose donor memory T-cell infusions after αβ T cell-depleted hematopoietic stem cell transplantation. Bone Marrow Transplant 56, 900–908 (2021). https://doi.org/10.1038/s41409-020-01128-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01128-2